Abstract
Purpose of Review
To highlight the recently approved therapeutic agents in psoriatic arthritis (PsA), drugs in the pipeline, as well as to discuss efficacy with regard to different clinical domains of PsA.
Recent Findings
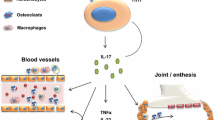
More than 15 years ago, tumor necrosis factor inhibitors (TNFi) were the first biologic disease modifying anti-rheumatic drugs (bDMARDs) that were approved for the treatment of PsA. Since then, multiple new therapeutic agents inhibiting other targets have emerged including biologics targeting interleukin (IL) 12/23, and IL 17 and oral agents targeting phosphodiesterase 4 (PDE4) and Janus kinases (JAKs). Many new agents with various modes of action including selective inhibition of IL 23, therapies promoting activated T cell apoptosis, inhibition of tyrosine kinase 2 (TYK2), and more are under active assessment in ongoing clinical trials.
Summary
Effective therapies for treating PsA have emerged over the last 15 years and newer agents continue to be discovered, allowing greater therapeutic options for controlling psoriatic disease activity and preventing joint damage and disability. Personalized therapy for patients with PsA is now a possibility.
Similar content being viewed by others
References
Papers of particular, interest published recently, have been highlighted as: • Of importance
• Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. 2017;376:2095–6. Psoriatic arthritis. This paper presents an updated and thorough review about the epidemiology, pathogenesis, and treatment of psoriatic arthritis.
• Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis rheumatol. 2016;68:1060–71. This paper summarizes the current treatment recommendations in psoriatic arthritis. It specifies treatment options and level of evidence for each domain (peripheral arthritis, enthesitis, dactylitis, skin, nail, and axial arthriits).
Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510.
Glintborg B, Ostergaard M, Krogh NS, Andersen MD, Tarp U, Loft AG, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor alpha inhibitor therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheum. 2013;65:1213–23.
Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DP, et al. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther. 2009;11:R52.
Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73:48–55.
Edwards CJ, Blanco FJ, Crowley J, Birbara CA, Jaworski J, Aelion J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065–73.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, Lespessailles E, Hall S, Hochfeld M, Hu C, Hough D, Stevens RM, Schett G. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020–26.
Cutolo M, Myerson GE, Fleischmann RM, Liote F, Diaz-Gonzalez F, Van den Bosch F, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724–34.
Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–9.
McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–9.
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373:1329–39.
McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–46.
Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87.
Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317–27.
Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295–306.
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–19.
Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–50.
• Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–36. This is a good example of a recent randomized controlled trial of a new therapeutic option in patients who have failed biologic theraoy for psoriatic arthritis.
Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550–58.
Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1705–13.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326–38.
Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson ADG, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523–532.
Deodhar A, Gottlieb A, Boehncke W-H, Dong B, Wang Y, Barchuk W, et al. OP0218 Efficacy and safety results of guselkumab, an anti-il23 monoclonal antibody, in patients with active psoriatic arthritis over 24 weeks: a phase 2a, randomized, double-blind, placebo-controlled study. Ann Rheum Dis. 2017;76(Suppl 2):142–3.
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551–60.
Feagan BG, Sandborn WJ, D'Haens G, Panes J, Kaser A, Ferrante M, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–709.
Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaci D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276–88.
Mease P, Genovese MC, Weinblatt M, Peloso PM, Chen K, Li Y, et al. Safety and efficacy of ABT-122, a TNF and IL-17–targeted dual variable domain (DVD)–Ig™, in psoriatic arthritis patients with inadequate response to methotrexate: results from a phase 2 trial. Arthritis Rheumatol. 2016; 68 (suppl 10). http://acrabstracts.org/abstract/safety-and-efficacy-of-abt-122-a-tnf-and-il-17-targeted-dual-variable-domain-dvd-ig-in-psoriatic-arthritis-patients-with-inadequate-response-to-methotrexate-results-from/. Accessed May 10, 2018.
Mease PJ, Gottlieb AB. Berman a, Drescher E, Xing J, Wong R, et al. The efficacy and safety of Clazakizumab, an anti-Interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 2016;68:2163–73.
Kavanaugh A, McInnes IB, Mease P, Krueger GG, Gladman D, van der Heijde D, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis. 2014;73:1689–94.
Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week 24 of the GO-VIBRANT study. Arthritis Rheumatol. 2017;69:2151–61.
Kavanaugh A, Kremer J, Ponce L, Cseuz R, Reshetko OV, Stanislavchuk M, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis. 2017;76:1009–19.
Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–75.
Robin-Jagerschmidt C LS, Marsais F, Ongenaert M, Monjardet A, Cauvin A, Saccomani C, et al. The JAK1 selective inhibitor filgotinib regulates both enthesis and colon inflammation in a mouse model of psoriatic arthritis [abstract]. Arthritis Rheumatol 2017; 69 (suppl 10). http://acrabstracts.org/abstract/the-jak1-selective-inhibitor-filgotinib-regulates-both-enthesis-and-colon-inflammation-in-a-mouse-model-of-psoriatic-arthritis/. Accessed January 13, 2018.
Genovese MC, Fleischmann R, Combe B, Hall S, Zhang Y, Zhou Y, Mohamed MEF, Meerwein S, Pangan AL. Upadacitinib (ABT-494) in Patients with Active Rheumatoid Arthritis and Inadequate Response or Intolerance to Biological Dmards: A Phase 3 Randomized, Placebo-Controlled, Double-Blind Study of a Selective JAK-1 Inhibitor [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). http://acrabstracts.org/abstract/upadacitinib-abt-494-in-patients-with-active-rheumatoid-arthritis-and-inadequate-response-or-intolerance-to-biological-dmards-a-phase-3-randomized-placebo-controlled-double-blind-study-of-a-selec/. Accessed May 9, 2018.
https://www.clinicaltrials.gov/ct2/show/NCT03104400?term=upadacitinib&cond=Psoriatic+Arthritis&rank=2. Accessed May 10 2018.
A phase II study evaluating the efficacy and safety of AbGn-168H in patients with active psoriatic arthritis. https://clinicaltrials.gov/ct2/show/NCT02267642?term=AbGn-168H&rank=5. Accessed January 3, 2018.
Phase IIa study of multiple doses of AbGn-168H by iv infusion in moderate to severe chronic plaque psoriasis patients (AbGn-168H). https://clinicaltrials.gov/ct2/show/NCT01855880?term=AbGn-168H&rank=3. Accessed January 3, 2018.
FDA approves inflectra, a biosimilar to remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 3, 2018.
A study to evaluate the safety, mode of action and clinical efficacy of GSK3050002 in subjects with psoriatic arthritis. NCT02671188 Accessed January 3,2018.
Single dose escalation trial to evaluate the safety, tolerability pharmacokinetics and pharmacodynamics of GSK3050002. NCT01984047 Accessed January 3, 2018.
Study to evaluate effectiveness and safety in subjects with moderate to severe psoriasis. NCT02931838 Accessed January 3, 2018.
FDA approves amjevita, a biosimilar to humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 3, 2018.
https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm494227.htm. Accessed May 10, 2018.
FDA approves erelzi, a biosimilar to enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 3, 2018.
Yoo DH, Racewicz A, Brzezicki J, Yatsyshyn R, Arteaga ET, Baranauskaite A, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2016;18:82.
Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–12.
Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177:1562–74.
Griffiths CEM, Thaci D, Gerdes S, Arenberger P, Pulka G, Kingo K, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176:928–38.
Nikiphorou E, Kautiainen H, Hannonen P, Asikainen J, Kokko A, Rannio T, et al. Clinical effectiveness of CT-P13 (infliximab biosimilar) used as a switch from remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Th. 2015;15:1677–83.
Gentileschi S, Barreca C, Bellisai F, Biasi G, Brizi MG, De Stefano R, et al. Switch from infliximab to infliximab biosimilar: efficacy and safety in a cohort of patients with different rheumatic diseases Response to: Nikiphorou E, Kautiainen H, Hannonen P, et al. Clinical effectiveness of CT-P13 (infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15:1677-1683. Expert Opin Biol Ther. 2016;16:1311–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Elalouf declares no conflict of interest.
Dr. Chandran reports personal fees from Amgen, grants and personal fees from Abbvie, grants and personal fees from Celgene, personal fees from Eli Lilly, personal fees from Janssen, personal fees from Novartis, personal fees from UCB, and personal fees from Pfizer, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of Topical Collection on Psoriatic Arthritis
Rights and permissions
About this article
Cite this article
Elalouf, O., Chandran, V. Novel Therapeutics in Psoriatic Arthritis. What Is in the Pipeline?. Curr Rheumatol Rep 20, 36 (2018). https://doi.org/10.1007/s11926-018-0746-0
Published:
DOI: https://doi.org/10.1007/s11926-018-0746-0