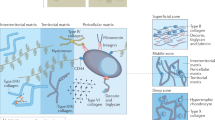
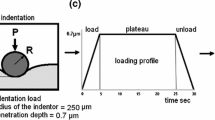
Abstract
Articular cartilage injuries and degenerative joint diseases are responsible for progressive pain and disability in millions of people worldwide, yet there is currently no treatment available to restore full joint functionality. As the tissue functions under mechanical load, an understanding of the physiologic or pathologic effects of biomechanical factors on cartilage physiology is of particular interest. Here, we highlight studies that have measured cartilage deformation at scales ranging from the macroscale to the microscale, as well as the responses of the resident cartilage cells, chondrocytes, to mechanical loading using in vitro and in vivo approaches. From these studies, it is clear that there exists a complex interplay among mechanical, inflammatory, and biochemical factors that can either support or inhibit cartilage matrix homeostasis under normal or pathologic conditions. Understanding these interactions is an important step toward developing tissue engineering approaches and therapeutic interventions for cartilage pathologies, such as osteoarthritis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mow VC, Proctor CS, Kelly MA. Biomechanics of Articular Cartilage. In: Nordin M, Frankel V, editors. Basic Biomechanics of the Muskuloskeletal system. 2nd ed. Philadelphia: Lea and Febiger; 1989.
Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916–26.
Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707.
Racunica TL, Teichtahl AJ, Wang Y, et al. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis Rheum. 2007;57(7):1261–8.
Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25.
Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):815–23.
Mow VC, Bachrach NM, Setton LA, et al. Stress, Strain, Pressure and Flow Fields in Articular Cartilage and Chondrocytes. In: Mow VC et al., editors. Cell Mechanics and Cellular Engineering. New York: Springer; 1994. p. 345–79.
Bischof JE, Spritzer CE, Caputo AM, et al. In vivo cartilage contact strains in patients with lateral ankle instability. J Biomech. 2010;43(13):2561–6.
Coleman JL, Widmyer MR, Leddy HA, et al. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46(3):541–7.
Widmyer MR, Utturkar GM, Leddy HA, et al. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013;65(10):2615–22.
Mosher TJ, Smith HE, Collins C, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234(1):245–9.
Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat. 2006;208(4):491–512.
Eckstein F, Tieschky M, Faber S, et al. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat Embryol (Berl). 1999;200(4):419–24.
Eckstein F, Tieschky M, Faber SC, et al. Effect of physical exercise on cartilage volume and thickness in vivo: MR imaging study. Radiology. 1998;207(1):243–8.
Liu F, Kozanek M, Hosseini A, et al. In vivo tibiofemoral cartilage deformation during the stance phase of gait. J Biomech. 2010;43(4):658–65.
Van de Velde SK, Bingham JT, Hosseini A, et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60(12):3693–702.
Bae WC, Lewis CW, Levenston ME, et al. Indentation testing of human articular cartilage: effects of probe tip geometry and indentation depth on intra-tissue strain. J Biomech. 2006;39(6):1039–47.
Chen AC, Bae WC, Schinagl RM, et al. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech. 2001;34(1):1–12.
Schinagl RM, Ting MK, Price JH, et al. Video microscopy to quantitate the inhomogeneous equilibrium strain within articular cartilage during confined compression. Ann Biomed Eng. 1996;24(4):500–12.
Choi JB, Youn I, Cao L, et al. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40(12):2596–603.
Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90(Pt 4):635–43.
Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res. 1987;5(4):509–22.
Haider MA, Schugart RC, Setton LA, et al. A mechano-chemical model for the passive swelling response of an isolated chondron under osmotic loading. Biomech Model Mechanobiol. 2006;5(2–3):160–71.
Vincent TL, Hermansson MA, Hansen UN, et al. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50(2):526–33.
Alexopoulos LG, Youn I, Bonaldo P, et al. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 2009;60(3):771–9.
Darling EM, Wilusz RE, Bolognesi MP, et al. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys J. 2010;98(12):2848–56.
Wilusz RE, Defrate LE, Guilak F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. J R Soc Interface. 2012;9(76):2997–3007.
Kim E, Guilak F, Haider MA. An axisymmetric boundary element model for determination of articular cartilage pericellular matrix properties in situ via inverse analysis of chondron deformation. J Biomech Eng. 2010;132(3):031011.
McLeod MA, Wilusz RE, Guilak F. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J Biomech. 2013;46(3):586–92.
Wilusz RE, Defrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol. 2012;31(6):320–7.
Wilusz RE, Guilak F. High resistance of the mechanical properties of the chondrocyte pericellular matrix to proteoglycan digestion by chondroitinase, aggrecanase, or hyaluronidase. J Mech Behav Biomed Mater. 2014;38:183–97.
Wilusz RE, Zauscher S, Guilak F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthr Cartil. 2013;21(12):1895–903.
Xu L, Golshirazian I, Asbury BJ, et al. Induction of high temperature requirement A1, a serine protease, by TGF-beta1 in articular chondrocytes of mouse models of OA. Histol Histopathol. 2014;29(5):609–18.
Guilak F, Hung CT. Physical Regulation of Cartilage Metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. 3rd ed. Philedalphia: Lippincott, Williams & Wilkins; 2004. p. 259–300.
Patwari P, Cheng DM, Cole AA, et al. Analysis of the relationship between peak stress and proteoglycan loss following injurious compression of human post-mortem knee and ankle cartilage. Biomech Model Mechanobiol. 2007;6(1–2):83–9.
Patwari P, Gaschen V, James IE, et al. Ultrastructural quantification of cell death after injurious compression of bovine calf articular cartilage. Osteoarthr Cartil. 2004;12(3):245–52.
Kurz B, Lemke A, Kehn M, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50(1):123–30.
Patwari P, Cook MN, DiMicco MA, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48(5):1292–301.
Kurz B, Jin M, Patwari P, et al. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19(6):1140–6.
Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205–12.
Quinn TM, Grodzinsky AJ, Hunziker EB, et al. Effects of injurious compression on matrix turnover around individual cells in calf articular cartilage explants. J Orthop Res. 1998;16(4):490–9.
Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18(4):391–9.
Buschmann MD, Kim YJ, Wong M, et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366(1):1–7.
Mauck RL, Soltz MA, Wang CC, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–60.
Ng KW, Mauck RL, Wang CC, et al. Duty Cycle of Deformational Loading Influences the Growth of Engineered Articular Cartilage. Cell Mol Bioeng. 2009;2(3):386–94.
Guilak F, Meyer BC, Ratcliffe A, et al. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthr Cartil. 1994;2(2):91–101.
Stolberg-Stolberg JA, Furman BD, Garrigues NW, et al. Effects of cartilage impact with and without fracture on chondrocyte viability and the release of inflammatory markers. J Orthop Res. 2013;31(8):1283–92.
Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36(5):780–92.
Chan PS, Schlueter AE, Coussens PM, et al. Gene expression profile of mechanically impacted bovine articular cartilage explants. J Orthop Res. 2005;23(5):1146–51.
Ashwell MS, Gonda MG, Gray K, et al. Changes in chondrocyte gene expression following in vitro impaction of porcine articular cartilage in an impact injury model. J Orthop Res. 2013;31(3):385–91.
Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33(12):1663–73.
Smith RL, Lin J, Trindade MC, et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37(2):153–61.
Smith RL, Rusk SF, Ellison BE, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;14(1):53–60.
O'Conor CJ, Leddy HA, Benefield HC, et al. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111(4):1316–21. This study identifies TRPV4-mediated intracellular Ca 2+ signaling as a key mechanosensitive pathway in articular chondrocytes and demonstrates its involvement in regulating chondrocyte ECM biosynthesis.
Phan MN, Leddy HA, Votta BJ, et al. Functional Characterization of TRPV4 as an Osmotically Sensitive Ion Channel in Porcine Articular Chondrocytes. Arthritis Rheum. 2009;60(10):3028–37.
Mobasheri A, Barrett-Jolley R Carter SD, et al. Functional Roles of Mechanosensitive Ion Channels, β1 Integrins and Kinase Cascades in Chondrocyte Mechanotransduction. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues: Moscow; 2005. http://www.ncbi.nlm.nih.gov/books/NBK7517/.
Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32(3):435–46.
Wann AKT, Zuo N, Haycraft CJ, et al. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012;26(4):1663–71. This work demonstrates a possible link between primary cilia in chondrocytes and ATP reception, and further establishes the role of the primary cilia in chondrocyte mechanotransduction.
Dolmetsch RE, Lewis RS, Goodnow CC, et al. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386(6627):855–8.
Ogawa H, Kozhemyakina E, Hung HH, et al. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014;28(2):127–39. This study identifies specific signaling pathways involved in the mechanical regulation of Prg4 expression in articular chondrocytes.
Bougault C, Aubert-Foucher E, Paumier A, et al. Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS One. 2012;7(5):e36964. Chondrocytes are shown to be highly responsive to mechanical stimulation, upregulating and downregulating a diverse array of genes.
Chowdhury TT, Appleby RN, Salter DM, et al. Integrin-mediated mechanotransduction in IL-1 beta stimulated chondrocytes. Biomech Model Mechanobiol. 2006;5(2–3):192–201.
Chai DH, Arner EC, Griggs DW, et al. Alphav and beta1 integrins regulate dynamic compression-induced proteoglycan synthesis in 3D gel culture by distinct complementary pathways. Osteoarthr Cartil. 2010;18(2):249–56.
Liang W, Ren K, Liu F, et al. Periodic mechanical stress stimulates the FAK mitogenic signal in rat chondrocytes through ERK1/2 activity. Cell Physiol Biochem. 2013;32(4):915–30.
Fitzgerald JB, Jin M, Dean D, et al. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279(19):19502–11.
Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthr Cartil. 2004;12(6):485–96.
Thompson CL, Chapple JP, Knight MM. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthr Cartil. 2014;22(3):490–8.
Saito T, Nishida K, Furumatsu T, et al. Histone deacetylase inhibitors suppress mechanical stress-induced expression of RUNX-2 and ADAMTS-5 through the inhibition of the MAPK signaling pathway in cultured human chondrocytes. Osteoarthr Cartil. 2013;21(1):165–74.
Lopez MJ, Kunz D, Vanderby Jr R, et al. A comparison of joint stability between anterior cruciate intact and deficient knees: a new canine model of anterior cruciate ligament disruption. J Orthop Res. 2003;21(2):224–30.
Arunakul M, Tochigi Y, Goetz JE, et al. Replication of chronic abnormal cartilage loading by medial meniscus destabilization for modeling osteoarthritis in the rabbit knee in vivo. J Orthop Res. 2013;31(10):1555–60.
Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr Cartil. 2007;15(9):1061–9.
Kuroki K, Cook CR, Cook JL. Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthr Cartil. 2011;19(9):1142–9.
Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–8.
Little CB, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–33.
Amiable N, Martel-Pelletier J, Lussier B, et al. Proteinase-activated receptor-2 gene disruption limits the effect of osteoarthritis on cartilage in mice: a novel target in joint degradation. J Rheumatol. 2011;38(5):911–20.
Clark AL, Votta BJ, Kumar S, et al. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62(10):2973–83.
Chang CF, Ramaswamy G, Serra R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthr Cartil. 2012;20(2):152–61. This study shows that loss of the chondrocyte primary cilia, a putative mechanotransducer in cartilage, leads to osteoarthritic changes.
Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391(Suppl(391 Suppl)):S108–15.
McNulty AL, Rothfusz NE, Leddy HA, et al. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31(7):1039–45.
Bougault C, Gosset M, Houard X, et al. Stress-Induced Cartilage Degradation Does Not Depend on the NLRP3 Inflammasome in Human Osteoarthritis and Mouse Models. Arthritis Rheum. 2012;64(12):3972–81.
Jovanovic D, Pelletier JP, Alaaeddine N, et al. Effect of IL-13 on cytokines, cytokine receptors and inhibitors on human osteoarthritis synovium and synovial fibroblasts. Osteoarthr Cartil. 1998;6(1):40–9.
Ushiyama T, Chano T, Inoue K, et al. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62(2):108–12.
Deschner J, Hofman CR, Piesco NP, et al. Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care. 2003;6(3):289–93.
Guilak F, Fermor B, Keefe FJ, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26.
Torzilli PA, Bhargava M, Chen CT. Mechanical Loading of Articular Cartilage Reduces IL-1-Induced Enzyme Expression. Cartilage. 2011;2(4):364–73. This study shows that applying mechanical load in combination with IL-1 can reduce the mRNA expression of matrix-degrading enzymes that would normally be upregulated in response to IL-1.
Torzilli PA, Bhargava M, Park S, et al. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthr Cartil. 2010;18(1):97–105.
Chowdhury TT, Bader DL, Lee DA. Dynamic compression inhibits the synthesis of nitric oxide and PGE(2) by IL-1beta-stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun. 2001;285(5):1168–74.
Chowdhury TT, Salter DM, Bader DL, et al. Signal transduction pathways involving p38 MAPK, JNK, NFkappaB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1beta and dynamic compression. Inflamm Res. 2008;57(7):306–13.
Xu Z, Buckley MJ, Evans CH, et al. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165(1):453–60.
Perera PM, Wypasek E, Madhavan S, et al. Mechanical signals control SOX-9, VEGF, and c-Myc expression and cell proliferation during inflammation via integrin-linked kinase, B-Raf, and ERK1/2-dependent signaling in articular chondrocytes. Arthritis Res Ther. 2010;12(3):R106. This study revealed a mechanism of differential kinase activation upstream of ERK1/2 phosphorylation that may regulate the effects of inflammation during mechanical loading in chondrocytes.
Nam J, Aguda BD, Rath B, et al. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS One. 2009;4(4):e5262.
Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthr Cartil. 2002;10(10):792–8.
Burleigh A, Chanalaris A, Gardiner MD, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64(7):2278–88. This study shows a strong link between OA progression and mechanical loading in a mouse model.
Aviezer D, Hecht D, Safran M, et al. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79(6):1005–13.
Bonassar LJ, Grodzinsky AJ, Frank EH, et al. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19(1):11–7.
Mauck RL, Nicoll SB, Seyhan SL, et al. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9(4):597–611.
Bonassar LJ, Grodzinsky AJ, Srinivasan A, et al. Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch Biochem Biophys. 2000;379(1):57–63.
Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3(6):e2341.
Allen JL, Cooke ME, Alliston T. ECM stiffness primes the TGFbeta pathway to promote chondrocyte differentiation. Mol Biol Cell. 2012;23(18):3731–42. This study shows that substrate stiffness and growth factors can have a synergistic response on chondrogenesis.
van der Kraan PM. Age-related alterations in TGF beta signaling as a causal factor of cartilage degeneration in osteoarthritis. Biomed Mater Eng. 2014;24:75–80.
Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol. 2013;13(3):449–54.
Acknowledgments
Supported in part by the Arthritis Foundation and NIH grants AR48182, AR48852, AG15768, AR50245, AG46927, and AG40868.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Johannah Sanchez-Adams reports the receipt of grants from the Arthritis Foundation.
Christopher J. O’Conor reports the receipt of grants from the NIH.
Farshid Guilak reports the receipt of grants from the Arthritis Foundation and the NIH.
Holly A. Leddy and Amy L. McNulty declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
About this article
Cite this article
Sanchez-Adams, J., Leddy, H.A., McNulty, A.L. et al. The Mechanobiology of Articular Cartilage: Bearing the Burden of Osteoarthritis. Curr Rheumatol Rep 16, 451 (2014). https://doi.org/10.1007/s11926-014-0451-6
Published:
DOI: https://doi.org/10.1007/s11926-014-0451-6