Abstract
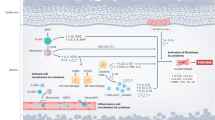
Efficient antifibrotic therapies are not available for patients with systemic sclerosis (SSc). This review summarizes the current preclinical and clinical evidence for imatinib and related tyrosine kinase inhibitors as potential antifibrotic therapies for SSc and other fibrotic diseases. In experimental models of SSc, imatinib, nilotinib, and dasatinib demonstrated strong antifibrotic effects. Imatinib not only prevented fibrosis in the bleomycin-induced model of dermal fibrosis and the tight skin mouse-1 model but also reduced established fibrosis in a modified bleomycin model. Open-label, proof-of-concept trials in SSc showed moderate effects on skin fibrosis, biological measures of skin fibrosis, and lung fibrosis compared with baseline measures. However, whether this reflects the natural course of the disease or is a result of treatment effects is unclear and needs to be analyzed in larger, multicenter, randomized, placebo-controlled trials. Toxicity is expected from cancer trials with frequent mild to moderate adverse events.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Varga J, Abraham D: Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 2007, 117:557–567.
Baroni SS, Santillo M, Bevilacqua F, et al.: Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 2006, 354:2667–2676.
Loizos N, Lariccia L, Weiner J, et al.: Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor alpha in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum 2009, 60:1145–1151.
Classen JF, Henrohn D, Rorsman F, et al.: Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum 2009, 60:1137–1144.
Dragun D, Distler JH, Riemekasten G, Distler O: Stimulatory autoantibodies to platelet-derived growth factor receptors in systemic sclerosis: what functional autoimmunity could learn from receptor biology. Arthritis Rheum 2009, 60:907–911.
Ludwicka A, Ohba T, Trojanowska M, et al.: Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J Rheumatol 1995, 22:1876–1883.
Yamakage A, Kikuchi K, Smith EA, et al.: Selective upregulation of platelet-derived growth factor alpha receptors by transforming growth factor beta in scleroderma fibroblasts. J Exp Med 1992, 175:1227–34.
Wahl SM: Transforming growth factor b: the good, the bad, and the ugly. J Exp Med 1994, 180:1587–1590.
Asano Y, Ihn H, Yamane K, et al.: Impaired Smad7-Smurf-mediated negative regulation of TGF-b signaling in scleroderma fibroblasts. J Clin Invest 2004, 113:253–264.
Asano Y, Ihn H, Yamane K, et al.: Increased expression of integrin avb5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 2006, 168:499–510.
Sonnylal S, Denton CP, Zheng B, et al.: Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum 2007, 56:334–344.
Bhattacharyya S, Ishida W, Wu M, et al.: A non-Smad mechanism of fibroblast activation by transforming growth factor-beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene 2009, 28:1285–1297.
Daniels CE, Wilkes MC, Edens M, et al.: Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 2004, 114:1308–1316.
Ciardiello F, Tortora G: EGFR antagonists in cancer treatment. N Engl J Med 2008, 358:1160–1174.
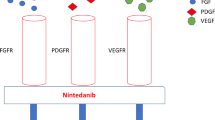
Distler JH, Distler O: Intracellular tyrosine kinases as novel targets for anti-fibrotic therapy in systemic sclerosis. Rheumatology (Oxford) 2008, 47(Suppl 5):v10–v11.
Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009, 54(1 Suppl):S10–19.
Deininger MW, O’Brien SG, Ford JM, et al.: Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol 2003, 21:1637–1647.
Quintas-Cardama A, Kantarjian H, Cortes J: Imatinib and beyond—exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol 2009, 6:535–543.
Bradeen HA, Edie CA, O’Hare T, et al.: Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyI-N nitrosourea (ENU)-based mutagenesis screen: High efficacy of drug combinations. Blood 2006, 108:2332–2338.
Distler JH, Jungel A, Huber LC, et al.: Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum 2007, 56:311–322.
Maurer B, Stanczyk J, Jungel A, et al.: MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 2010, 62:1733–1743.
Pannu J, Asano Y, Nakerakanti S, Smith E, et al.: Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum 2008, 58:2528–2537.
Asano Y: Future treatments in systemic sclerosis. J Dermatol 2010, 37:54–70.
Beyer C, Schett G, Distler O, Distler JH: Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum 2010, 62:2831–2844.
Akhmetshina A, Dees C, Pileckyte M, et al.: Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J 2008, 22:2214–2222.
Abdollahi A, Li M, Ping G, et al.: Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005, 201:925–935.
Wang S, Wilkes MC, Leof EB, et al.: Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J 2005, 19:1–11.
Yoshiji H, Noguchi R, Kuriyama S, et al.: Imatinib mesylate (STI-571) attenuates liver fibrosis development in rats. Am J Physiol Gastrointest Liver Physiol 2005, 288:G907–913.
• Akhmetshina A, Venalis P, Dees C, et al.: Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum 2009, 60:219–224. This study demonstrated the efficacy of imatinib in Tsk-1 mice representing later, less inflammatory stages of SSc and showed the efficacy of imatinib in an animal model of established fibrosis.
Chaudhary NI, Schnapp A, Park JE: Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 2006, 173:769–776.
Beham-Schmid C, Apfelbeck U, Sill H, et al.: Treatment of chronic myelogenous leukemia with the tyrosine kinase inhibitor STI571 results in marked regression of bone marrow fibrosis. Blood 2002, 99:381–383.
Bueso-Ramos CE, Cortes J, Talpaz M, et al.: Imatinib mesylate therapy reduces bone marrow fibrosis in patients with chronic myelogenous leukemia. Cancer 2004, 10:332–336.
• Magro L, Mohty M, Catteau B, et al.: Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood 2009, 114:719–722. Published in the same issue as the article by Olivieri et al. [34•], this article shows encouraging results for cGvHD in an open-label study with imatinib.
• Olivieri A, Locatelli F, Zecca M, et al.: Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood 2009, 114:709–718. This article also shows encouraging results for cGvHD in an open-label study with imatinib.
Nakasone H, Kanda Y, Takasaki H , et al.; Kanto Study Group for Cell Therapy: Prophylactic impact of imatinib administration after allogeneic stem cell transplantation on the incidence and severity of chronic graft versus host disease in patients with Philadelphia chromosome-positive leukemia. Leukemia 2010, 24:1236–1239.
Stadler M, Ahlborn R, Kamal H, et al.: Limited efficacy of imatinib in severe pulmonary chronic graft-versus-host disease. Blood 2009, 114:3718–3719.
Sabnani I, Zucker MJ, Rosenstein ED, et al.: A novel therapeutic approach to the treatment of scleroderma-associated pulmonary complications: safety and efficacy of combination therapy with imatinib and cyclophosphamide. Rheumatology (Oxford) 2009, 48:49–52.
Sfikakis PP, Gorgoulis VG, Katsiari CG, et al.: Imatinib for the treatment of refractory, diffuse systemic sclerosis. Rheumatology (Oxford) 2008, 47:735–737.
van Daele PL, Dik WA, Thio HB, et al.: Is imatinib mesylate a promising drug in systemic sclerosis? Arthritis Rheum 2008, 58:2549–2552.
Chung L, Fiorentino DF, Benbarak MJ, et al.: Molecular framework for response to imatinib mesylate in systemic sclerosis. Arthritis Rheum 2009, 60:584–591.
Kay J, High WA: Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum 2008, 58:2543–2548.
Distler JH, Manger B, Spriewald BM, et al.: Treatment of pulmonary fibrosis for twenty weeks with imatinib mesylate in a patient with mixed connective tissue disease. Arthritis Rheum 2008, 58:2538–2542.
Spiera RF, Gordon JK, Mersten J, et al.: Imatinib Mesylate (Gleevec™) in the Treatment of Diffuse Cutaneous Systemic Sclerosis: Results of a One Year, Phase IIa, Single Arm, Open Label Clinical Trial. [abstract 2192]. Presented at the 74th Annual Scientific Meeting of American College of Rheumatology. Atlanta, GA; November 7–11, 2010.
Saggar R, Khanna D, Mayes MD, et al.: Open Labeled Study Of Imatinib Mesylate (Gleevec) In The Treatment Of Systemic Sclerosis- Associated Active Interstitial Lung Disease (SSc-ILD): Preliminary Results. Am J Respir Crit Care Med 2010, 181:A3991.
Pope J, McBain D, Petrlich L, et al.: A randomized double blind proof of concept trial of imatinib (Gleevec) in active diffuse scleroderma (dSSc). Ann Rheum Dis 2010, 69(Suppl3):410.
Distler O, Distler JH, Varga J, et al.: A Multi-Center, Open-Label, Proof of Concept Study of Imatinib Mesylate Demonstrates No Benefit for the Treatment of Fibrosis in Patients with Early, Diffuse Systemic Sclerosis. [abstract 560]. Presented at the 74th Annual Scientific Meeting of the American College of Rheumatology. Atlanta, GA; November 7–11, 2010.
• Daniels CE, Lasky JA, Limper AH, et al.: Imatinib-IPF Study Investigators: Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med 2010, 181:604–610. This trial was the first randomized, placebo-controlled trial to assess the efficacy of imatinib in the treatment of fibrosis.
Widmer N, Decosterd LA, Csajka C, et al.: Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol 2006, 62:97–112.
Azuma M, Nishioka Y, Aono Y, et al.: Role of alpha1-acid glycoprotein in therapeutic antifibrotic effects of imatinib with macrolides in mice. Am J Respir Crit Care Med 2007, 176:1243–1250.
Maurer B, Busch N, Jungel A, et al.: Tyrosine Kinase Inhibitors (TKI) Are Promising Therapeutic Agents for the Proliferative Vasculopathy in SSc [abstract]. Arthritis Rheum 2009, 60 Suppl 10:1263.
Disclosure
Dr. Jörg Distler has served as a consultant for Actelion Pharmaceuticals, Pfizer, and GlaxoSmithKline; has received grant support from Novartis, Bayer Schering Pharma, Cell Gen Therapeutics, NicOx, Array BioPharma, Bristol-Myers Squibb, and Ergonex Pharma GmbH; and has received payment for development of educational presentations (including service on speakers’ bureaus) from Actelion Pharmaceuticals, Pfizer, GlaxoSmithKline, and Bayer Schering Pharma.
Dr. Oliver Distler has served as a consultant for Actelion Pharmaceuticals, Pfizer, FibroGen, Ergonex Pharma GmbH, Bristol-Myers Squibb, Sanofi-Aventis, and United BioSource Corp.; has received grant support from Pfizer and Ergonex Pharma GmbH; has received payment for development of educational presentations (including service on speakers’ bureaus) from Actelion Pharmaceuticals, Pfizer, and Medac; and has had travel/accommodations covered or reimbursed by UCB. Dr. Iwamoto reported no conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwamoto, N., Distler, J.H.W. & Distler, O. Tyrosine Kinase Inhibitors in the Treatment of Systemic Sclerosis: From Animal Models to Clinical Trials. Curr Rheumatol Rep 13, 21–27 (2011). https://doi.org/10.1007/s11926-010-0142-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-010-0142-x