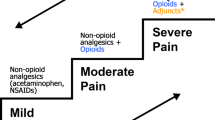
Abstract
Purpose of Review
Headaches are common in children and adolescents. Treatments for debilitating migraine are often not FDA approved or lack evidence of efficacy for children. This narrative review looks at the evidence for acute and preventative pharmacologic and non-pharmacologic treatment of pediatric migraine, as well as reviewing any recent or ongoing clinical trials.
Recent Findings
Studies have been published on pharmacological treatments for headache, as well as non-pharmacological treatments. Recent findings in pediatric migraine using onabotulinumtoxinA, calcitonin gene related peptide antagonists, interventional procedures, and devices are reviewed.
Summary
Pharmacologic as well as non-pharmacologic approaches for the prevention and treatment of migraine show safety and efficacy data that is promising. These treatments should be incorporated in a multi-modal approach to the management of pediatric migraine. Continued studies, prospective and randomized, are needed to further assess these newer treatments for migraine in the pediatric setting.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
•• Oskoui M, Pringsheim T, Holler-Managan Y, Potrebic S, Billinghurst L, Gloss D, et al. Practice guideline update summary: acute treatment of migraine in children and adolescents: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2019;93(11):487–99. https://doi.org/10.1212/WNL.0000000000008095. Epub 2019 Aug 14. Erratum in: Neurology. 2020;94(1):50. This is a practice guideline for pediatric migraine supported by the American Headache Society and the American Academy of Neurology.
Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurol. 2010;9(2):190–204. https://doi.org/10.1016/S1474-4422(09)70303-5.
Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting relating to acute gastroenteritis in children and adolescents. Cochrane Database Sys Rev. 2011;CD005506.
Winner P, Linder S, Hershey AD. Consistency of response to sumatriptan/naproxen sodium in a randomized placebo-controlled, cross-over study for the acute treatment of migraine in adolescence. Headache. 2015;55:519–28. https://doi.org/10.1111/head.12555.
Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297:1443–54. https://doi.org/10.1001/jama.297.13.1443.
Ashina S, Terwindt GM, Steiner TJ, Lee MJ, Porreca F, Tassorelli C, et al. Medication overuse headache. Nat Rev Dis Primers. 2023;9(1):5. https://doi.org/10.1038/s41572-022-00415-0.
Burckart GJ, Kim C. The revolution in pediatric drug development and drug use: therapeutic orphans no more. J Pediatr Pharmacol Ther. 2020;25(7):565–73. https://doi.org/10.5863/1551-6776-25.7.565.
•• Oskoui M, Pringsheim T, Billinghurst L, Potrebic S, Gersz EM, Gloss D, et al. Practice guideline update summary: pharmacologic treatment for pediatric migraine prevention: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2019;93(11):500–9. https://doi.org/10.1212/WNL.0000000000008105. Epub 2019 Aug 14. Erratum in: Neurology. 2020;94(1):50. This is a practice guideline for pediatric migraine supported by the American Headache Society and the American Academy of Neurology.
Li BUK. Managing cyclic vomiting syndrome in children: beyond the guidelines. Eur J Pediatr. 2018;177(10):1435–42. https://doi.org/10.1007/s00431-018-3218-7. Epub 2018 Aug 3.
Tekin H, Edem P. Effects and side effects of migraine prophylaxis in children. Pediatr Int. 2022;64(1):e15094. https://doi.org/10.1111/ped.15094.
Banerjee S, Butcher R. Pharmacological interventions for chronic pain in pediatric patients: a review of guidelines [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563527.
Rajdev S, Wassmer E, Agrawal S, et al. Efficacy and tolerability of the use gabapentin for migraine prophylaxis in children. Arch Dis Child. 2012;97:A140.
Powers SW, Coffey CS, Chamberlin LA, et al. CHAMP investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med 2017;376(2):115–24. https://doi.org/10.1056/NEJMoa1610384.
Powers SW, Hershey AD, Coffey CS, et al. The childhood and adolescent migraine prevention (CHAMP) study: a report on baseline characteristics of participants. Headache. 2016;56:859–70. https://doi.org/10.1111/head.12810.
•• Powers SW, Coffey CS, Chamberlin LA, et al. Prevalence of headache days and disability 3 years after participation in the childhood and adolescent migraine prevention medication trial. JAMA Netw Open. 2021;4(7):e2114712. https://doi.org/10.1001/jamanetworkopen.2021.14712. This is a follow-up survey-based study which was conducted following the conclusion of the CHAMP trial, one of the largest and most important randomized controlled study in the pediatric population.
Shah S, Calderon MD, Wu W, Grant J, Rinehart J. OnabotulinumtoxinA (Botox®) for prophylactic treatment of pediatric migraine: a retrospective longitudinal analysis. J Child Neurol. 2018;33(9):580–6. https://doi.org/10.1177/0883073818776142. Epub 2018 Jun 7.
Kabbouche M, O’Brien H, Hershey AD. OnabotulinumtoxinA in pediatric chronic daily headache. Curr Neurol Neurosci Rep. 2012;12(2):114–7. https://doi.org/10.1007/s11910-012-0251-1.
Winner PK, Kabbouche M, Yonker M, Wangsadipura V, Lum A, Brin MF. A randomized trial to evaluate onabotulinumtoxinA for prevention of headaches in adolescents with chronic migraine. Headache. 2020;60(3):564–75. https://doi.org/10.1111/head.13754. Epub 2020 Feb 9.
Marcelo R, Freund B. The efficacy of botulinum toxin in pediatric chronic migraine: a literature review. J Child Neurol. 2020;35(12):844–51. https://doi.org/10.1177/0883073820931256. Epub 2020 Jun 24.
Goenka A, Yu SG, George MC, Chikkannaiah M, MacDonald S, Stolfi A, et al. Is Botox right for me: when to assess the efficacy of the Botox injection for chronic migraine in pediatric population. Neuropediatrics. 2022. https://doi.org/10.1055/a-1832-9168. Epub 2022 Apr 22.
Shah S, Calderon MD, Crain N, Pham J, Rinehart J. Effectiveness of onabotulinumtoxinA (Botox) in pediatric patients experiencing migraines: a randomized, double-blinded, placebo-controlled crossover study in the pediatric pain population. Reg Anesth Pain Med. 2021;46(1):41–8. https://doi.org/10.1136/rapm-2020-101605. Epub 2020 Oct 26.
Ali SS, Bragin I, Rende E, Mejico L, Werner KE. Further evidence that onabotulinum toxin is a viable treatment option for pediatric chronic migraine patients. Cureus. 2019;11(3):e4343. https://doi.org/10.7759/cureus.4343.
Peck J, Zeien J, Patel M, Cornett EM, Berger AA, Hasoon J et al. Review of interventional therapies for refractory pediatric migraine. Health Psychol Res. 2023;10(5):1–8. https://doi.org/10.52965/001c.67853.
Szperka CL, Gelfand AA, Hershey AD. Patterns of use of peripheral nerve blocks and trigger point injections for pediatric headache; results of a survey of the American Headache Society Pediatric and Adolescent Section. Headache. 2016;1597–607. https://doi.org/10.1111/head.12939.
Gelfand AA, Reider AC, Goadsby PJ. Outcomes of greater occipital nerve injections in pediatric patients with chronic primary headache disorders. Pediatr Neurol. 2014;50:135–9. https://doi.org/10.1016/j.pediatrneurol.2013.09.008. Epub 2013 Nov 20.
Puledda F, Goadsby PJ, Prabhakar P. Treatment of disabling headache with greater nerve injections in a large population of childhood and adolescent patients: a service evaluation. J Headache Pain. 2018;19:5. https://doi.org/10.1186/s10194-018-0835-5.
Goenka A, Chikkannaiah M, Fonseca LD, Kuman G. Peripheral nerve blocks: a tool for inpatient status migrainosus. Pediatr Neurol. 2023;138:81–6. https://doi.org/10.1016/j.pediatrneurol.2022.10.010. Epub 2022 Oct 28.
Hassan R, Gudiwala V, Dawn P, Jeyes L. Greater occipital nerve block as an effective intervention for medically refractory pediatric migraine: a retrospective study. Cureus. 2023;15(2):e34930. https://doi.org/10.7759/cureus.34930.
Mumber HE, Szperka CL. Retrospective study of nerve blocks for the treatment of headaches in children & adolescents. Headache. 2016;56:37.
Renaudon-Smith E, Toolis C, Goadsby P, Prabhakar P. Greater occipital nerve injection (GONI) for chronic headache in children. J Headache Pain. 2010;11(Suppl 1):S76.
Dubrovsky AS, Friedman D, Kocilowicz H. Pediatric post-traumatic headaches and peripheral nerve blocks of the scalp: a case series and patient satisfaction survey. Headache. 2014;54:878–87. https://doi.org/10.1111/head.12334.
Seegar TA, Orr S, Bodell L, Lockyer L, Rajapakse T, Barlow KM. Occipital nerve blocks for pediatric posttraumatic headache: a case series: Journal of Child Neurology. 2015;30:1142–6. https://doi.org/10.1177/0883073814553973. Epub 2014 Nov 18.
Zaremski JL, Hurley RW, Herman D, Bauer RM, Ahn AH. Poster 182 occipital neuralgia blockade as a treatment after concussion. A case series PM&R. 2014;6(9):S249. https://doi.org/10.1016/j.pmrj.2014.08.576.
Dance L, Aria D, Schaefer C, Kaye R, Yonker M, Towbin R. Safety and efficacy of sphenopalatine ganglion blockade in children: initial experience. J Vasc Interv Radiol. 2017;282:2(Supplement S8).
Mousa MA, Aria DJ, Mousa AA, Schaefer CM, Temkit MHH, Towbin RB. Sphenopalatine ganglion nerve block for the treatment of migraine headaches in the pediatric population. Pain Physician. 2021;24(1):E111–6.
Kouri M, Somaini M, Cardenas VHG, Niburski K, Vigouroux M, Ingelmo P. Transnasal sphenopalatine ganglion block for the preventive treatment of chronic daily headaches in adolescents. Children. 2021;8:606. https://doi.org/10.3390/children8070606.
Turner SB, Szperka CL, Hershey AD, Law EF, Palermo TM, Groenewald CB. Association of headache with school functioning among children and adolescents in the United States. JAMA Pediatr. 2021;175(5):522–4. https://doi.org/10.1001/jamapediatrics.2020.5680.
Farber AJ, Lagman-Bartolome AM, Rajapaske T. Drugs for the acute treatment of migraine in children and adolescents. Paediatri Child Health. 2017;22(8):454–8. https://doi.org/10.1093/pch/pxx170.
Esparham A, Boorigie M, Ablatt S, Connelly M, Bickel J. Improving acute treatment of pediatric primary headache disorders with a novel headache treatment center: retrospective review of preliminary outcomes. J Child Neurol. 2021;36(1):54–9. https://doi.org/10.1177/0883073820952997.
Hershey AD, Lin T, Gruper Y, et al. Remote electrical neuromodulation for acute treatment of migraine in adolescents. Headache. 2021;61(2):310–7. https://doi.org/10.1111/head.14042.
Irwin SL, Qubty W, Allen E, Patniyot I, Goadsby PJ, Gelfand AA. Transcranial magnetic stimulation for migraine prevention in adolescents: a pilot open-label study. Headache. 2018. https://doi.org/10.1111/head.13284.
Grazzi L, Egeo G, Liebler E, Padovan AM, Barbanti P, et al. Non-invasive vagus nerve stimulation (nVNS) as symptomatic treatment of migraine in young patients: a preliminary safety study. Neurol Sci. 2017;38(Suppl 1):197–9. https://doi.org/10.1007/s10072-017-2942-5.
Hargreaves R, Olesen J. Calcitonin gene-related peptide modulators - the history and renaissance of a new migraine drug class. Headache. 2019;59(6):951–70. https://doi.org/10.1111/head.13510. Epub 2019 Apr 25.
•• Szperka CL, VanderPluym J, Orr SL, et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache. 2018;58(10):1658–69. https://doi.org/10.1111/head.13414. This is a very important expert opinion paper on use of some of the newest treatments for pediatric migraine as there is no clinical trial data available in this population.
Evers S. CGRP in childhood and adolescence migraine: (patho)physiological and clinical aspects. Curr Pain Headache Rep. 2022;26(6):475–80. https://doi.org/10.1007/s11916-022-01047-5.
Gibbler RC, Knestrick KE, Reidy BL, Lax DN, Powers SW. Management of chronic migraine in children and adolescents: where are we in 2022? Pediatric Health Med Ther. 2022;13:309–23. https://doi.org/10.2147/PHMT.S334744.
Greene KA, Gentile CP, Szperka CL, et al. Calcitonin gene-related peptide monoclonal antibody use for the preventive treatment of refractory headache disorders in adolescents. Pediatr Neurol. 2021;114:62–7. https://doi.org/10.1016/j.pediatrneurol.2020.09.014.
Szkutnik-Fiedler D. Pharmacokinetics, pharmacodynamics and drug-drug interactions of new anti-migraine drugs-lasmiditan, gepants, and calcitonin-gene-related peptide (CGRP) receptor monoclonal antibodies. Pharmaceutics. 2020;12(12):1180. https://doi.org/10.3390/pharmaceutics12121180.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–32. https://doi.org/10.1056/NEJMoa1705848.
Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–22. https://doi.org/10.1056/NEJMoa1709038.
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–21. https://doi.org/10.1212/WNL.0000000000006640. Epub 2018 Nov 16.
Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–54. https://doi.org/10.1177/0333102420905132. Epub 2020 Feb 19.
Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737–45. https://doi.org/10.1016/S0140-6736(19)31606-X. Epub 2019 Jul 13.
Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10268):51–60. https://doi.org/10.1016/S0140-6736(20)32544-7. Epub 2020 Dec 15.
Ailani J, Lipton RB, Goadsby PJ, Guo H, Miceli R, Severt L, et al. Atogepant for the preventive treatment of migraine. N Engl J Med. 2021;385(8):695–706. https://doi.org/10.1056/NEJMoa2035908.
Vila-Pueyo M. Targeted 5-HT1F therapies for migraine. Neurotherapeutics. 2018;15(2):291–303. https://doi.org/10.1007/s13311-018-0615-6.
Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894–904. https://doi.org/10.1093/brain/awz134.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Reena Rastogi has served on the advisory board for Theranica Bio-electronics Ltd. and received honoraria. Dr. Rastogi is also a sub-investigator for Biohaven Pharmaceuticals, Inc. and Eli Lilly and Company. Dr. Eric Hastriter is a sub-investigator for Biohaven Pharmaceuticals, Inc. and Eli Lilly and Company. Dr. Kavitha Karnik is a sub-investigator for Biohaven Pharmaceuticals, Inc. and Eli Lilly and Company. Dr. Robert Little is a principal investigator for Biohaven Pharmaceuticals, Inc. and Eli Lilly and Company. Dr. Kara Lewis is a sub-investigator for Biohaven Pharmaceuticals, Inc. and Eli Lilly and Company.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rastogi, R.G., Hastriter, E.V., Evans, R.L. et al. Advances in the Acute and Preventive Treatment of Pediatric Migraine. Curr Pain Headache Rep 27, 521–529 (2023). https://doi.org/10.1007/s11916-023-01157-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-023-01157-8