Abstract
Neuromodulation is a promising, novel approach for the treatment of primary headache disorders. Neuromodulation offers a new dimension in the treatment that is both easily reversible and tends to be very well tolerated. The autonomic nervous system is a logical target given the neurobiology of common primary headache disorders, such as migraine and the trigeminal autonomic cephalalgias (TACs). This article will review new encouraging results of studies from the most recent literature on neuromodulation as acute and preventive treatment in primary headache disorders, and cover some possible underlying mechanisms. We will especially focus on vagus nerve stimulation (VNS) and sphenopalatine ganglion (SPG) since they have targeted autonomic pathways that are cranial and can modulate relevant pathophysiological mechanisms. The initial data suggests these approaches will find an important role in headache disorder management going forward.
Similar content being viewed by others
Introduction
Headache disorders are the most common form of disability on a global basis and the sixth most common cause of disability worldwide [1••]. The cumulative lifetime incidence of migraine approaches 50 % in females if one includes probable migraine and chronic migraine [2], while the 1 year prevalence for cluster headache, for which there is no cumulative data, is about 0.1 % of the population [3, 4]. Although the majority of people affected by primary headache disorders can be classified as episodic, a percentage of patients develop chronic forms often resistant to regular pharmacological treatment, which result in an enormous burden for sufferers and difficulties for physicians. Estimated societal burdens run to billions in the USA [5] and Europe [6••]. On the other hand, even patients with less severe headache syndromes can develop noticeable side effects with medical therapy and therefore require a constant pursuit for new treatment options.
These growingly recognized problems have led to the expansion of an exciting new branch of headache treatment: neuromodulation. This group of techniques comprises non-invasive treatments which, by targeting the central or peripheral nervous system, aim at modifying pain and other mechanisms involved in headache, and more invasive surgical approaches directed towards structures directly involved in the genesis of specific headache syndromes. Here, we have been tasked to cover neuromodulation of autonomic pathways plausibly intersecting with migraine [7, 8••] and trigeminal autonomic cephalalgia (TAC, [9••]) neurobiology.
Non-invasive Vagus Nerve Stimulation (nVNS)
Background
VNS has been used as treatment for epilepsy [10, 11] and depression [12] for many years. Invasive devices have dominated use and are variably accepted in clinical practice. The vagus nerve is the tenth cranial nerve. It is a mixed sensory and motor nerve with a long course and many functions, which is paired. It shall be referred to in the singular unless laterality is relevant. It arises from or converges upon
-
a.
The dorsal motor nucleus of the vagus (DMNX): parasympathetic visceral efferents
-
b.
The nucleus ambiguous: parasympathetic cardiac preganglionic fibres
-
c.
The nucleus of the solitary tract (NTS): afferent taste and visceral afferents
-
d.
The spinal trigeminal nucleus: ear, posterior fossa dura and larynx.
It is considered to be substantially an afferent nerve in terms of identified fibres, with between 65 and 80 % being sensory, although this often cited figure is based on feline data [13]. Upon exiting the medulla oblongata, it descends and exits the cranium through the jugular foramen. The nerve conveys parasympathetic preganglionic fibres widely and returns limited cutaneous sensory and widespread visceral afferent traffic. It is notable that the right vagal nerve innervates the sinoatrial node to slow the heart rate, while the left vagus innervates the atrioventricular node.
VNS and Trigeminal Pain
A bundle of afferent fibres of the vagus nerve, along with the glossopharyngeal and facial nerve fibres innervating the ear and the larynx, terminates in the trigeminal nucleus caudalis [14]. Furthermore, the nucleus tractus solitarius—the main nucleus of the vagus nerve—has shown to receive dural nociceptive afferents [15, 16]. Physiological studies demonstrated an effect of vagal afferents on non-cranial nociceptive pathways [17–19]. Vagal stimulation can modulate the pial blood flow [20]. However, acute vagotomy in experimental animals does not alter craniovascular responses due to sphenopalatine ganglion activation [21]. Most recent studies in rats demonstrate that vagus nerve stimulation can reduce pain and allodynia [22, 23] in the trigeminal basin. This may be mediated by an ascending antinociceptive effect of the vagus nerve on the second order neurons of the spinothalamic and spinoreticular tract responsible for the spinal nociceptive transmission to the trigeminal nuclear complex [24, 25]. One suggested mechanism is a reduction in the glutamate levels and of neuronal firing in the spinal trigeminal nucleus secondary to continuous vagus stimulation [22]. Notably, no cardiac side effects were reported in any of the studies, even though they are theoretically possible based on the efferent projections of the nucleus ambiguous to the preganglionic parasympathetic cardiac neurons. This could be due to the pulse wave of vagal nerve stimulator devices that are specifically designed to preferentially activate A- and B-myelinated fibres and not parasympathetic C fibres of the vagus nerve [26, 27].
Migraine
Initial convincing attention of a possible effect of VNS in patients came from an epileptic patient implanted with a VNS device, whose epilepsy was not responsive but that noted a reduction in migraine headache shortly after the beginning of the treatment [28]. Sometime later, VNS devices were implanted in patients with refractory headache without epilepsy with some limited success [29]. Similarly, two other seizure cohorts have shown improvements in migraine with implanted VNS, although with some change in seizure frequency [30, 31], thus making it difficult to infer causality in this case. Lastly, a useful effect on migraine has been reported in patients with VNS for depression who also had improvements in migraine [32]. These early reports provided some context for the clinical studies on a nVNS.
Recently, a portable transcutaneous non-invasive device that stimulates the cervical portion of the vagus nerve has been developed (GammaCore®), with animal studies demonstrating that its effects are similar to those of implanted stimulators [33]. The nVNS is administered by placing the device on the neck, which then produces a mild electrical current that is transmitted to the vagus nerve through the skin [34••]. The treatment has been used in primary headaches with very promising results and a high safety and tolerability rate.
Acute Attack Treatment with nVNS
The first large pilot study to investigate nVNS in migraine was an open-label single-arm trial aimed at treating acute attacks [35]. In this study, 27 patients with episodic migraine treated 80 attacks with two unilateral 90-s doses, separated by 15-min intervals. Of the 54 moderate or severe attacks, 22 % were completely aborted at 2 h, while 43 % had a significant reduction in pain scores. This effect is comparable to that of similarly tolerated triptans. Side effects were generally mild, infrequent and well tolerated; the ones more clearly associated with the treatment itself were neck twitching, raspy voice and redness over the application site on the neck. A further open-label study was conducted to treat headache worsenings in patients with chronic migraine [36]. Twenty-two patients, 18 females, treated 79 attacks, with ≥50 % reduction in VAS at 2 h in 46 % of patients. Another recent open-label, single-arm, multicenter study investigated the use of nVNS for the acute treatment of high-frequency episodic and chronic migraine. A total of 131 attacks were treated by 48 patients with two unilateral 120-s doses of nVNS at 3-min intervals. At 2 h, the pain-relief rate was 51.1 % and the pain-free rate was 22.9 %. The positive response to the device was more evident in the subgroup of patients with lower frequency of attacks [37].
Preventive Treatment with nVNS
Regarding prevention, initial results with VNS seem quite promising. In a small Belgian study, 18 patients—12 with migraine—were treated with transcutaneous VNS. In total, ten discontinued the treatment because of lack of efficacy and/or side effects, although one patient with medication overuse had a reduction in more than 50 % of headache frequency [38]. Silberstein and colleagues recently performed a double-blind, sham-controlled pilot study using nVNS as a preventive in chronic migraine. The treatment—two 90-s doses given three times a day—was performed in the blinded phase on 59 patients for 2 months and was followed by a 6-month open-label phase. At the end of the 2-month double-blind phase, there was a −1.9 day (n = 26) reduction in headache days in the active and a 0.2 day (n = 23) change in headache days in sham (p = 0.12) [39], leaving open a question of how long one should treat to achieve neuromodulation. Another randomized sham-controlled study for the prevention of episodic migraine is ongoing and currently recruiting patients (NCT02378844).
Cluster Headache (CH)
Two CH patients were reported to benefit from an implanted vagus nerve stimulator [29]. Following the availability of nVNS and its increased use, an audit of 19 patients with active episodic (n = 8) or chronic (n = 11) CH was reviewed over a 12-month period [40•]. Patients were instructed to administer up to three consecutive doses for the acute treatment of an attack, whereas for the preventive use, they were to self-administer 2–3 consecutive doses twice a day. The treatments were given for 120 s unilaterally to the side of the headache; the intensity of the stimulation was controlled by the patient. Results were encouraging: 79 % of patients reported an overall improvement of their initial conditions of approximately 50 %. In these “responders”, around half of the attacks were aborted in less than 15 min, and the attack frequency was also reduced of nearly 50 % respect to baseline during the treatment period. No serious adverse events were reported, and the treatment showed good tolerability in most patients.
In a recent prospective multicenter randomized controlled trial, nVNS was compared to the standard of care in the preventive treatment of chronic cluster headache [41]. Data from the 93 patients included in the analysis showed CH attacks were significantly reduced of 7.6 per week with nVNS treatment. This consisted of three stimulations twice daily for preventive treatment, as well as optional acute treatments for attack rescue. Furthermore, patients used a lower amount of rescue medications and showed good safety and tolerability with nVNS. Recently, a multicenter, double-blind, randomized controlled study to evaluate the efficacy and safety of acute CH treatment with nVNS has been completed (NCT01792817), as well as a study on the acute treatment of episodic and chronic cluster headaches (NCT01958125). Results of these trials are eagerly awaited. Finally, one CCH patient from the Belgian cohort previously mentioned had a significant attack reduction with prophylactic tVNS. Two patients from the same group were diagnosed with hemicrania continua, one of which had an initial decrease in pain intensity with tVNS followed by a quick relapse; results are not available for the second patient [38].
Sphenopalatine Ganglion (SPG) Stimulation
Background
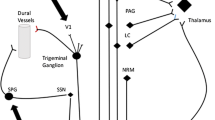
The sphenopalatine (pterygopalatine) ganglion [42, 43] is a major outflow pathway for the facial (VIIth) nerve cranial dilator system [44, 45], which is the efferent portion of the trigeminal-autonomic reflex [46]. This system arises from neurons in the superior salivatory nucleus [47] that receive inputs from trigeminal nucleus caudalis [48]. The SPG is a hexamethonium-sensitive nicotinic ganglion [21] containing vasoactive intestinal polypeptide (VIP) [49, 50], pituitary adenylate cyclase-activating peptide (PACAP) [51] and nitric oxide synthase [52]. This pathway is the basis for canonical cranial autonomic symptoms such as lacrimation, conjunctival injection, nasal symptoms, aural symptoms and periorbital oedema, when activated typically by trigeminal nociceptive afferents [53•]. Thus, experimentally induced pain [54], migraine [55, 56] and the trigeminal autonomic cephalalgias (TACs) [46] all share expression of the pathway, with a remarkable differentiation in prominence, reproducibility and lateralization of the symptoms in TACs [57].
Maizels [58, 59] demonstrated the effectiveness of nasal lidocaine-induced SPG block in reducing pain during migraine attacks. The first trial to attempt SPG stimulation for migraine treatment was a small pilot study performed by Tepper and colleagues [60], who applied electrical stimulation via a needle inserted in the sphenopalatine fossa through an infrazygomatic approach in 11 patients with refractory migraine. Induced attacks were aborted (n = 2) or relieved (n = 3) in only five patients, although the authors discuss that this relatively low response could be due to incorrect lead placement or concomitant medication overuse headache in most patients. At the moment, an RCT is testing the efficacy of an implanted microstimulator in migraine (NCT01540799), and another trial for the acute treatment of episodic migraine (NCT01294046) has been completed and not reported. A single case report of intractable facial pain presenting some migrainous features and treated with SPG modulation has also been published [61].
Cluster Headache
Medically refractory cluster headache is a truly awful problem. Currently, such patients would be offered occipital nerve stimulation—ONS—[62, 63], although this approach still requires controlled evidence for its efficacy [64]. Patients have been offered deep-brain stimulation in the region of the posterior hypothalamic grey [65] based on earlier neuroimaging work [66]. Deep-brain stimulation failed its initial controlled trial [67] and has an established, albeit small, mortality [68], while ONS is disorder non-specific; better approaches are clearly needed.
Initial attempts at SPG manipulation involved ablation and nerve blocks, which were not particularly effective and carried the drawbacks of an invasive approach [69–72]. More recently, a small proof of concept study with five cluster headache patients examined the effect of percutaneous stimulation of the SPG in treating acute attacks, with positive results. The treatment, delivered through a removable electrode, caused a complete abortion in 11 of the 18 treated attacks as well as the resolution of cranial autonomic symptoms, when these were present [73].
Based on these encouraging findings, a miniaturized implantable neurostimulator was developed, containing a lead with six electrodes that is implanted in the pterygopalatine fossa close to the SPG and anchored to the zygomatic process of the maxilla [74••]. The device is controlled remotely by the physician or the patient, who can adjust the intensity based on the voltage at which deep paresthesias are evoked behind the root of the nose, indicating correct activation [75]. The efficacy and safety of this device were examined in a European-based randomized controlled trial in 28 refractory chronic cluster headache patients who received either active, sub-perception or sham stimulation [74••]. Of the 566 treated attacks, pain relief was achieved in 67 % of the attacks treated with full stimulation (of which the average frequency was 120 Hz) compared to 7 % in both sham and sub-threshold stimulation. Of the 28 patients, 19 (68 %) benefitted significantly from the treatment, with an effective acute attack response in 9 (32 %) as well as an unexpected frequency reduction in 12 (43 %). The most common side effects of treatment were sensory disturbances and pain secondary to the surgical implantation, which generally resolved completely. A long-term follow-up at 18 months is being completed, and preliminary data showed a sustained therapeutic benefit for 66 % of patients [76]. At the moment, a trial is enrolling to explore these positive results in the acute treatment of chronic CH (NCT02168764).
Conclusions
The armamentarium for the treatment of migraine and the trigeminal autonomic cephalalgias is rapidly expanding thanks to neuromodulation techniques. The newer methods seem much better tolerated and offer important therapeutic benefits. Equally attractive in many ways is that bench-based understanding is being applied to neuromodulation to yield bedside advances in treatment. Clinicians can look forward to the results of a number of ongoing studies and the real possibility to add these exciting methods to their practice.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800.
Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–8.
Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28:614–8.
Sjaastad O, Bakketeig LS. Cluster headache prevalence. Vaga study of headache epidemiology. Cephalalgia. 2003;23:528–33.
Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–54.
Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19:155–62. Cost of headache disorders compared to other brain disorders in Europe.
Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346:257–70.
Akerman S, Holland P, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570–84. Comprehensive review of migraine basic mechanisms.
Goadsby PJ, Lipton RB. A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with autonomic features, including new cases. Brain. 1997;120:193–209. First description of the concept of Trigeminal-Autonomic Cephalalgias (TACs).
Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2000;55(8):1167–71.
Schachter SC. Vagus nerve stimulation therapy summary: five years after FDA approval. Neurology. 2002;59(6 Suppl 4):S15–20.
12. Yuan TF, Li A, Sun X, Arias-Carrion O, Machado S. Vagus nerve stimulation in treating depression: a tale of two stories. Curr Mol Med. 2015.
Foley JO, DuBois FS. Quantitative studies of the vagus nerve in the cat. J Comp Neurol. 1937;67:49–67.
Ruggiero DA, Underwood MD, Mann JJ, Anwar M, Arango V. The human nucleus of the solitary tract: visceral pathways revealed with an “in vitro” postmortem tracing method. J Auton Nerv Syst. 2000;79:181–90.
Kaube H, Keay KA, Hoskin KL, Bandler R, Goadsby PJ. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993;629:95–102.
Goadsby PJ, Hoskin KL. The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: a c-fos immunocytochemical study. J Anat. 1997;190:367–75.
Ren K, Randich A, Gebhart GF. Vagal afferent modulation of spinal nociceptive transmission in the rat. J Neurophysiol. 1989;62(2):401–15.
Randich A, Ren K, Gebhart GF. Electrical stimulation of cervical vagal afferents. II. Central relays for behavioral antinociception and arterial blood pressure decreases. J Neurophysiol. 1990;64(4):1115–24.
Ren K, Randich A, Gebhart GF. Electrical stimulation of cervical vagal afferents. I. Central relays for modulation of spinal nociceptive transmission. J Neurophysiol. 1990;64(4):1098–114.
Forbes HS, Nason GI, Wortman RC. Cerebral circulation XLIV: vasodilation in the pia following stimulation of the vagus aortic and carotid sinus nerves. Arch Neurol Psychiatr. 1937;37:334–50.
Goadsby PJ, Lambert GA, Lance JW. Effects of locus coeruleus stimulation on carotid vascular resistance in the cat. Brain Res. 1983;278:175–83.
Lyubashina OA, Sokolov AY, Panteleev SS. Vagal afferent modulation of spinal trigeminal neuronal responses to dural electrical stimulation in rats. Neuroscience. 2012;222:29–37.
Oshinsky ML, Murphy AL, Hekierski Jr H, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155(5):1037–42.
Bossut DF, Maixner W. Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. Pain. 1996;65(1):101–9.
Nishikawa Y, Koyama N, Yoshida Y, Yokota T. Activation of ascending antinociceptive system by vagal afferent input as revealed in the nucleus ventralis posteromedialis. Brain Res. 1999;833(1):108–11.
Castoro MA, Yoo PB, Hincapie JG, Hamann JJ, Ruble SB, Wolf PD, et al. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol. 2011;227(1):62–8.
Yoo PB, Lubock NB, Hincapie JG, Ruble SB, Hamann JJ, Grill WM. High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J Neural Eng. 2013;10(2):026003.
Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia. 2002;22:482–4.
Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82–6.
Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain. 2003;4:530–4.
Lenaerts ME, Oommen KJ, Couch JR, Skaggs V. Can vagus nerve stimulation help migraine? Cephalalgia. 2008;28:392–5.
Cecchini AP, Mea E, Tullo V, Curone M, Franzini A, Broggi G, et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci. 2009;30 Suppl 1:S101–4.
Hoffmann TJ, Simon BJ, Zhang Y, Emala CW. Low voltage vagal nerve stimulation reduces bronchoconstriction in guinea pigs through catecholamine release. Neuromodulation. 2012;15(6):527–36.
Martelletti P, Jensen RH, Antal A, Arcioni R, Brighina F, de Tommaso M, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14(1):86. Important statement from the European Headache Federation on neuromodulation in primary headache disorders.
Goadsby PJ, Grosberg BM, Mauskop A, Cady R. Effect of non-invasive vagus nerve stimulation on acute migraine: an open label pilot study. Cephalalgia. 2014;34:986–93.
Moscato D, Moscato FR, Liebler EJ. Efficacy of noninvasive vagus nerve stimulation (nVNS) in the treatment of acute migraine attacks. Headache. 2014;44:1418.
Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 2015;16:61.
Magis D, Gerard P, Schoenen J. Transcutaneous Vagus Nerve Stimulation (tVNS) for headache prophylaxis: initial experience. J Headache Pain. 2013;14 Suppl 1:198.
Silberstein SD, Da Silva AN, Calhoun AH, Grosberg BM, Lipton RB, Cady RK, et al. Non-invasive Vagus Nerve Stimulation for Chronic Migraine Prevention in a Prospective, Randomized, Sham-Controlled Pilot Study (the EVENT Study): report from the double-blind phase. Headache. 2014;54:1426.
Nesbitt AD, Marin JCA, Tompkins E, Ruttledge MH, Goadsby PJ. Initial experience with a novel non-invasive vagus nerve stimulation device for the treatment of cluster headache. Neurology (Minneap). 2015;84:1–5. First published experience of noninvasive vagal nerve stimulation in cluster headache.
Gaul C, Diener HC, Silver N, Magis D, Reuter U, Andersson A, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia. 2015;21.
Gray H. Anatomy of the human body (www.bartleby.com/107/). Philadelphia: Lea & Febiger; 1918.
Goadsby PJ, Lambert GA, Lance JW. The peripheral pathway for extracranial vasodilatation in the cat. J Auton Nerv Syst. 1984;10:145–55.
Goadsby PJ. Effect of stimulation of the facial nerve on regional cerebral blood flow and glucose utilization in cats. Am J Physiol. 1989;257:R517–R21.
Goadsby PJ. Characteristics of facial nerve elicited cerebral vasodilatation determined with laser Doppler flowmetry. Am J Physiol. 1991;260:R255–R62.
Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1:37–43.
Knight YE, Classey JD, Lasalandra MP, Akerman S, Kowacs F, Hoskin KL, et al. Patterns of fos expression in the rostral medulla and caudal pons evoked by noxious craniovascular stimulation and periaqueductal gray stimulation in the cat. Brain Res. 2005;1045:1–11.
Spencer SE, Sawyer WB, Wada H, Platt KB, Loewy AD. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res. 1990;534:149–69.
Goadsby PJ, Macdonald GJ. Extracranial vasodilatation mediated by VIP (vasoactive intestinal polypeptide). Brain Res. 1985;329:285–8.
Goadsby PJ, Shelley S. High frequency stimulation of the facial nerve results in local cortical release of vasoactive intestinal polypeptide in the anesthetised cat. Neurosci Lett. 1990;112:282–9.
Uddman R, Goadsby PJ, Jansen I, Edvinsson L. PACAP, a VIP-like peptide, immunohistochemical localization and effect upon cat pial arteries and cerebral blood flow. J Cereb Blood Flow Metab. 1993;13:291–7.
Edvinsson L, Mulder H, Goadsby PJ, Uddman R. Calcitonin gene-related peptide and nitric oxide in the trigeminal ganglion: cerebral vasodilatation from trigeminal nerve stimulation involves mainly calcitonin gene-related peptide. J Auton Nerv Syst. 1998;70:15–22.
May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiological implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19:115–27. Review of the physiology of the trigeminal-autonomic reflex.
Frese A, Evers S, May A. Autonomic activation in experimental trigeminal pain. Cephalalgia. 2003;23:67–8.
Obermann M, Yoon M-S, Dommes P, Kuznetsova J, Maschke M, Weimar C, et al. Prevalence of trigeminal autonomic symptoms in migraine: a population-based study. Cephalalgia. 2007;27:504–9.
Lai T-H, Fuh J-L, Wang S-J. Cranial autonomic symptoms in migraine: characteristics and comparison with cluster headache. J Neurol Neurosurg Psychiatry. 2009;80:1116–9.
Goadsby PJ. Lacrimation, conjunctival injection, nasal symptoms…cluster headache, migraine and cranial autonomic symptoms in primary headache disorders—what’s new? J Neurol Neursourg Psychiatry. 2009;80:1057–8.
Maizels M, Geiger AM. Intranasal lidocaine for migraine: a randomized trial and open-label follow-up. Headache. 1999;39:543–51.
Maizels M. Intranasal lidocaine to prevent headache following migraine aura. Headache. 1999;39:439–42.
Tepper SJ, Rezai A, Narouze S, Steiner C, Mohajer P, Ansarinia M. Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache. 2009;49(7):983–9.
Elahi F, Reddy CG. Sphenopalatine ganglion electrical nerve stimulation implant for intractable facial pain. Pain Physician. 2015;18(3):E403–9.
Burns B, Watkins L, Goadsby PJ. Successful treatment of medically intractable cluster headache using occipital nerve stimulation (ONS). Lancet. 2007;369:1099–106.
Burns B, Watkins L, Goadsby PJ. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology. 2009;72:341–5.
Wilbrink LA, Teernstra OP, Haan J, van Zwet EW, Evers SM, Spincemaille GH, et al. Occipital nerve stimulation in medically intractable, chronic cluster headache. The ICON study: rationale and protocol of a randomised trial. Cephalalgia. 2013;33(15):1238–47.
Leone M, Proietti Cecchini A, Franzini A, Broggi G, Cortelli P, Montagna P, et al. Lessons from 8 years’ experience of hypothalamic stimulation in cluster headache. Cephalalgia. 2008;28:787–97.
May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275–8.
Fontaine D, Lazorthes Y, Mertens P, Blond S, Geraud G, Fabre N, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11:23–31.
Schoenen J, Di Clemente L, Vandenheede M, Fumal A, De Pasqua V, Mouchamps M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128:940–7.
Devoghel JC. Cluster headache and sphenopalatine block. Acta Anaesthesiol Belg. 1981;32(1):101–7.
Felisati G, Arnone F, Lozza P, Leone M, Curone M, Bussone G. Sphenopalatine endoscopic ganglion block: a revision of a traditional technique for cluster headache. Laryngoscope. 2006;116:1447–50.
Yang LY, Oraee S. A novel approach to transnasal sphenopalatine ganglion injection. Pain Physician. 2006;9(2):131–4.
Narouze S, Kapural L, Casanova J, Mekhail N. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache. 2009;49:571–7.
Ansarinia M, Rezai A, Tepper SJ, Steiner CP, Stump J, Stanton-Hicks M, et al. Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache. 2010;50(7):1164–74.
Schoenen J, Jensen RH, Lanteri-Minet M, Lainez JM, Gaul C, Goodman AM, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment—pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33:816–30. First demonstration of the efficacy of sphenopalatine ganglion stimulation in cluster headache.
Narouze SN. Role of sphenopalatine ganglion neuroablation in the management of cluster headache. Curr Pain Headache Rep. 2010;14(2):160–3.
Lainez J, May A, Gaul C, Schoenen J, Goodman A, Caparso A, et al. Sphenopalatine ganglion (SPG) stimulation in the pathway CH-1 study reduces headache burden in patients before and after sustained periods of cluster attack remission. Cephalalgia. 2015;35(6S):80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Francesca Puledda declares no conflict of interest. Peter J. Goadsby declares grant support and personal fees from Allergan, eNeura Inc. and Amgen Inc.; he also declares personal fees from Autonomic Technologies Inc., Alder Biopharmaceuticals, Pfizer Inc., Dr. Reddy’s Laboratories, Zosano Pharma Corporation, Colucid Pharmaceuticals, Ltd., Eli-Lilly and Company, Avanir Pharmaceuticals, WL Gore & Associates, Heptares Therapeutics, Nupathe Inc., Teva, Cipla Ltd., Ajinomoto Pharmaceuticals Co., Akita Biomedical, Wells Fargo, Ethicon, USA, EMKinetics, Promius Pharma, Supernus and medicoegal work; and personal fees from Journal Watch and Up-to-Date for editorial work. He also declares personal fees and stock options (no commercial product) from Trigemina Inc. He also declares a patent pending for magnetic stimulation for headache.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuromodulation
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Puledda, F., Goadsby, P.J. Current Approaches to Neuromodulation in Primary Headaches: Focus on Vagal Nerve and Sphenopalatine Ganglion Stimulation. Curr Pain Headache Rep 20, 47 (2016). https://doi.org/10.1007/s11916-016-0577-5
Published:
DOI: https://doi.org/10.1007/s11916-016-0577-5