Abstract
Purpose of Review
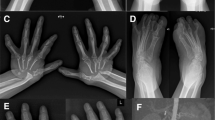
Multicentric carpotarsal osteolysis (MCTO) is an ultra-rare disorder characterized by osteolysis of the carpal and tarsal bones, subtle craniofacial deformities, and nephropathy. The molecular pathways underlying the pathophysiology are not well understood.
Recent Findings
MCTO is caused by heterozygous mutations in MAFB, which encodes the widely expressed transcription factor MafB. All MAFB mutations in patients with MCTO result in replacement of amino acids that cluster in a phosphorylation region of the MafB transactivation domain and account for a presumed gain-of-function for the variant protein. Since 2012, fewer than 60 patients with MCTO have been described with 20 missense mutations in MAFB. The clinical presentations are variable, and a genotype-phenotype correlation is lacking. Osteolysis, via excessive osteoclast activity, has been regarded as the primary mechanism, although anti-resorptive agents demonstrate little therapeutic benefit.
Summary
This paper appraises current perspectives of MafB protein action, inflammation, and dysfunctional bone formation on the pathogenesis of the skeletal phenotype in MCTO. More research is needed to understand the pathogenesis of MCTO to develop rational therapies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Tsunakawa Y, Hamada M, Matsunaga Y, Fuseya S, Jeon H, Wakimoto Y, et al. Mice harboring an MCTO mutation exhibit renal failure resembling nephropathy in human patients. Exp Anim 2019;68(1):103-11. https://doi.org/10.1538/expanim.18-0093. This paper reports the generation of a mouse harboring a human MCTO mutation using CRISPR/Cas9 technology. The mice develop a renal phenotype that resembles nephropathy in patients with MCTO.
• Wu J, Wang L, Xu Y, Zhang Z, Yan X, An Y, et al. Multicentric carpo-tarsal osteolysis syndrome mimicking juvenile idiopathic arthritis: two case reports and review of the literature. Front Pediatr 2021;9:745812. Overview of the clinical and genetic features of 51 published cases of genetically confirmed MCTO.
Wang PW, Eisenbart JD, Cordes SP, Barsh GS, Stoffel M, Le Beau MM. Human KRML (MAFB): cDNA cloning, genomic structure, and evaluation as a candidate tumor suppressor gene in myeloid leukemias. Genomics. 1999;59(3):275–81.
•• Zankl A, Duncan EL, Leo PJ, Clark GR, Glazov EA, Addor MC, et al. Multicentric carpotarsal osteolysis is caused by mutations clustering in the amino-terminal transcriptional activation domain of MAFB. Am J Hum Genet 2012;90(3): 494-501. This paper identified MAFB as the genetic basis for MCTO.
Tsuchiya M, Misaka R, Nitta K, Tsuchiya K. Transcriptional factors, Mafs and their biological roles. World J Diabetes. 2015;6(1):175–83. https://doi.org/10.4239/wjd.v6.i1.175.
Katsuoka F, Yamamoto M. Small Maf proteins (MafF, MafG, MafK): history, structure and function. Gene. 2016;586(2):197–205.
Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994;14(1):700–12.
Wu Z, Nicoll M, Ingham RJ. AP-1 family transcription factors: a diverse family of proteins that regulate varied cellular activities in classical hodgkin lymphoma and ALK+ ALCL. Exp Hematol Oncol. 2021;10(1):4.
Takahashi S. Functional analysis of large MAF transcription factors and elucidation of their relationships with human diseases. Exp Anim. 2021;70(3):264–71.
Mehawej C, Courcet JB, Baujat G, Mouy R, Gerard M, Landru I, et al. The identification of MAFB mutations in eight patients with multicentric carpo-tarsal osteolysis supports genetic homogeneity but clinical variability. Am J Med Genet A 2013;161a(12): 3023-3029.
Zhuang L, Adler S, Aeberli D, Villiger PM, Trueb B. Identification of a MAFB mutation in a patient with multicentric carpotarsal osteolysis. Swiss Med Wkly. 2017;147:w14529.
Chen K, Zamariolli M, Soares MFF, Meloni VA, Melaragno MI. Multicentric carpotarsal osteolysis syndrome in a mother and daughter with a MAFB missense variant and natural history of the disease. Mol Syndromol. 2022;13(1):50–5.
Li J, Shi L, Lau K, Ma Y, Jia S, Gao X. Identification of a novel mutation in the MAFB gene in a pediatric patient with multicentric carpotarsal osteolysis syndrome using next-generation sequencing. Eur J Med Genet. 2020;63(6):103902.
Mumm S, Huskey M, Duan S, Wenkert D, Madson KL, Gottesman GS, et al. Multicentric carpotarsal osteolysis syndrome is caused by only a few domain-specific mutations in MAFB, a negative regulator of RANKL-induced osteoclastogenesis. Am J Med Genet A 2014;164a(9): 2287-2293.
Choochuen P, Rojneuangnit K, Khetkham T, Khositseth S. The first report of multicentric carpotarsal osteolysis syndrome caused by MAFB mutation in Asian. Case Rep Med 2018: 6783957.
Upadia J, Gomes A, Weiser P, Descartes M. A familial case of multicentric carpotarsal osteolysis syndrome and treatment outcome. J Pediatr Genet. 2018;7(4):174–9.
Regev R, Sochett EB, Elia Y, Laxer RM, Noone D, Whitney-Mahoney K, Filipowski K, Shamas A, Vali R. Multicentric carpotarsal osteolysis syndrome (MCTO) with generalized high bone turnover and high serum RANKL: Response to denosumab. Bone Rep. 2021;14:100747.
Dworschak GC, Draaken M, Hilger A, Born M, Reutter H, Ludwig M. An incompletely penetrant novel MAFB (p.Ser56Phe) variant in autosomal dominant multicentric carpotarsal osteolysis syndrome. Int J Mol Med. 2013;32(1):174–8.
Sun K, Barlow B, Malik F, Inglis A, Figgie M, Goodman S. Total hip arthroplasty in a patient with multicentric carpotarsal osteolysis: a case report. Hss J. 2016;12(2):177–81.
Park PG, Kim KH, Hyun HS, Lee CH, Park JS, Kie JH, Choi YH, Moon KC, Cheong HI. Three cases of multicentric carpotarsal osteolysis syndrome: a case series. BMC Med Genet. 2018;19(1):164.
• Närhi A, Fernandes A, Toiviainen-Salo S, Harris J, McInerney-Leo A, Lazarus S, et al. A family with partially penetrant multicentric carpotarsal osteolysis due to gonadal mosaicism: first reported case. Am J Med Genet A 2021;185(8): 2477-2481. First reported case of partially penetrant MCTO due to genetic mosaicism.
Stajkovska A, Mehandziska S, Stavrevska M, Jakovleva K, Nikchevska N, Mitrev Z, Kungulovski I, Zafiroski G, Tasic V, Kungulovski G. Trio clinical exome sequencing in a patient with multicentric carpotarsal osteolysis syndrome: first case report in the Balkans. Front Genet. 2018;9:113.
Ma NS. Symptoms of multicentric carpotarsal osteolysis respond to anti-inflammatory treatment. 2020 Annual Meeting of the American Society for Bone and Mineral Research Virtual Event September 11-15, 2020. J Bone Miner Res 2020 Nov;35 Suppl 1:S1-S349. https://doi.org/10.1002/jbmr.4206.
Han SI, Aramata S, Yasuda K, Kataoka K. MafA stability in pancreatic beta cells is regulated by glucose and is dependent on its constitutive phosphorylation at multiple sites by glycogen synthase kinase 3. Mol Cell Biol. 2007;27(19):6593–605. https://doi.org/10.1128/MCB.01573-06.
Rocques N, Abou Zeid N, Sii-Felice K, Lecoin L, Felder-Schmittbuhl MP, Eychene A, et al. GSK-3-mediated phosphorylation enhances Maf-transforming activity. Mol Cell. 2007;28(4):584–97. https://doi.org/10.1016/j.molcel.2007.11.009.
Herath NI, Rocques N, Garancher A, Eychene A, Pouponnot C. GSK3-mediated MAF phosphorylation in multiple myeloma as a potential therapeutic target. Blood Cancer J. 2014;4:e175. https://doi.org/10.1038/bcj.2013.67.
Niceta M, Stellacci E, Gripp KW, Zampino G, Kousi M, Anselmi M, Traversa A, Ciolfi A, Stabley D, Bruselles A, Caputo V, Cecchetti S, Prudente S, Fiorenza MT, Boitani C, Philip N, Niyazov D, Leoni C, Nakane T, et al. Mutations impairing GSK3-mediated MAF phosphorylation cause cataract, deafness, intellectual disability, seizures, and a down syndrome-like facies. Am J Hum Genet. 2015;96(5):816–25. https://doi.org/10.1016/j.ajhg.2015.03.001.
Bialkowska AB, Liu Y, Nandan MO, Yang VW. A colon cancer-derived mutant of Kruppel-like factor 5 (KLF5) is resistant to degradation by glycogen synthase kinase 3beta (GSK3beta) and the E3 ubiquitin ligase F-box and WD repeat domain-containing 7alpha (FBW7alpha). J Biol Chem. 2014;289(9):5997–6005. https://doi.org/10.1074/jbc.M113.508549.
He P, Yang JW, Yang VW, Bialkowska AB. Kruppel-like factor 5, Increased in pancreatic ductal adenocarcinoma, promotes proliferation, acinar-to-ductal metaplasia, pancreatic intraepithelial neoplasia, and tumor growth in mice. Gastroenterology. 2018;154(5):1494–508 e13. https://doi.org/10.1053/j.gastro.2017.12.005.
Chen Q, Dowhan DH, Liang D, Moore DD, Overbeek PA. CREB-binding protein/p300 co-activation of crystallin gene expression. J Biol Chem. 2002;277(27):24081–9.
Guo S, Burnette R, Zhao L, Vanderford NL, Poitout V, Hagman DK, Henderson E, Özcan S, Wadzinski BE, Stein R. The stability and transactivation potential of the mammalian MafA transcription factor are regulated by serine 65 phosphorylation. J Biol Chem. 2009;284(2):759–65. https://doi.org/10.1074/jbc.M806314200.
Benkhelifa S, Provot S, Nabais E, Eychene A, Calothy G, Felder-Schmittbuhl MP. Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol Cell Biol. 2001;21(14):4441–52. https://doi.org/10.1128/MCB.21.14.4441-4452.2001.
Guo S, Vanderford NL, Stein R. Phosphorylation within the MafA N terminus regulates C-terminal dimerization and DNA binding. J Biol Chem. 2010;285(17):12655–61. https://doi.org/10.1074/jbc.M110.105759.
He Y, Wang S, Tong J, Jiang S, Yang Y, Zhang Z, Xu Y, Zeng Y, Cao B, Moran MF, Mao X. The deubiquitinase USP7 stabilizes Maf proteins to promote myeloma cell survival. J Biol Chem. 2020;295(7):2084–96. https://doi.org/10.1074/jbc.RA119.010724.
Giudicelli F, Gilardi-Hebenstreit P, Mechta-Grigoriou F, Poquet C, Charnay P. Novel activities of Mafb underlie its dual role in hindbrain segmentation and regional specification. Dev Biol. 2003;253(1):150–62. https://doi.org/10.1006/dbio.2002.0864.
Vazquez-Echeverria C, Dominguez-Frutos E, Charnay P, Schimmang T, Pujades C. Analysis of mouse kreisler mutants reveals new roles of hindbrain-derived signals in the establishment of the otic neurogenic domain. Dev Biol. 2008;322(1):167–78. https://doi.org/10.1016/j.ydbio.2008.07.025.
Conrad E, Dai C, Spaeth J, Guo M, Cyphert HA, Scoville D, Carroll J, Yu WM, Goodrich LV, Harlan DM, Grove KL, Roberts CT Jr, Powers AC, Gu G, Stein R. The MAFB transcription factor impacts islet alpha-cell function in rodents and represents a unique signature of primate islet beta-cells. Am J Physiol Endocrinol Metab. 2016;310(1):E91–E102. https://doi.org/10.1152/ajpendo.00285.2015.
Iacovazzo D, Flanagan SE, Walker E, Quezado R, de Sousa Barros FA, Caswell R, Johnson MB, Wakeling M, Brändle M, Guo M, Dang MN, Gabrovska P, Niederle B, Christ E, Jenni S, Sipos B, Nieser M, Frilling A, Dhatariya K, et al. MAFA missense mutation causes familial insulinomatosis and diabetes mellitus. Proc Natl Acad Sci USA. 2018;115(5):1027–32. https://doi.org/10.1073/pnas.1712262115.
Hang Y, Stein R. MafA and MafB activity in pancreatic beta cells. Trends Endocrinol Metab. 2011;22(9):364–73. https://doi.org/10.1016/j.tem.2011.05.003.
Miyatsuka T, Matsuoka TA, Kaneto H. Transcription factors as therapeutic targets for diabetes. Expert Opin Ther Targets. 2008;12(11):1431–42.
Kamitani-Kawamoto A, Hamada M, Moriguchi T, Miyai M, Saji F, Hatamura I, Nishikawa K, Takayanagi H, Hitoshi S, Ikenaka K, Hosoya T, Hotta Y, Takahashi S, Kataoka K. MafB interacts with Gcm2 and regulates parathyroid hormone expression and parathyroid development. J Bone Miner Res. 2011;26(10):2463–72. https://doi.org/10.1002/jbmr.458.
Sadl V, Jin F, Yu J, Cui S, Holmyard D, Quaggin S, et al. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol. 2002;249(1):16–29. https://doi.org/10.1006/dbio.2002.0751.
Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, Kudo T, Nagata M, Greaves DR, Engel JD, Yamamoto M, Takahashi S. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26(15):5715–27. https://doi.org/10.1128/mcb.00001-06.
Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS. Defining the molecular character of the developing and adult kidney podocyte. PLoS One. 2011;6(9):e24640. https://doi.org/10.1371/journal.pone.0024640.
Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109(8):3253–9. https://doi.org/10.1182/blood-2006-09-048249.
Howell K, Posluszny J, He LK, Szilagyi A, Halerz J, Gamelli RL, Shankar R, Muthu K. High MafB expression following burn augments monocyte commitment and inhibits DC differentiation in hemopoietic progenitors. J Leukoc Biol. 2012;91(1):69–81. https://doi.org/10.1189/jlb.0711338.
Cuevas VD, Anta L, Samaniego R, Orta-Zavalza E, Vladimir de la Rosa J, Baujat G, et al. MAFB determines human macrophage anti-inflammatory polarization: relevance for the pathogenic mechanisms operating in multicentric carpotarsal osteolysis. J Immunol 2017;198(5):2070-2081. https://doi.org/10.4049/jimmunol.1601667.
Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15(12):731–44. https://doi.org/10.1038/nri3920.
Sarrazin S, Mossadegh-Keller N, Fukao T, Aziz A, Mourcin F, Vanhille L, Kelly Modis L, Kastner P, Chan S, Duprez E, Otto C, Sieweke MH. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell. 2009;138(2):300–13. https://doi.org/10.1016/j.cell.2009.04.057.
Soucie EL, Weng Z, Geirsdottir L, Molawi K, Maurizio J, Fenouil R, et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science 2016;351(6274):aad5510. https://doi.org/10.1126/science.aad5510.
Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, Bergsagel PL, Kuehl WM, Staudt LM. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5(2):191–9. https://doi.org/10.1016/s1535-6108(04)00019-4.
Morito N, Yoh K, Fujioka Y, Nakano T, Shimohata H, Hashimoto Y, Yamada A, Maeda A, Matsuno F, Hata H, Suzuki A, Imagawa S, Mitsuya H, Esumi H, Koyama A, Yamamoto M, Mori N, Takahashi S. Overexpression of c-Maf contributes to T-cell lymphoma in both mice and human. Cancer Res. 2006;66(2):812–9. https://doi.org/10.1158/0008-5472.CAN-05-2154.
Niceta M, Barbuti D, Gupta N, Ruggiero C, Tizzano EF, Graul-Neumann L, Barresi S, Nishimura G, Valenzuela I, López-Grondona F, Fernandez-Alvarez P, Leoni C, Zweier C, Tzschach A, Stellacci E, del Fattore A, Dallapiccola B, Zampino G, Tartaglia M. Skeletal abnormalities are common features in Aymé-Gripp syndrome. Clin Genet. 2020;97(2):362–9.
Alkhunaizi E, Koenekoop RK, Saint-Martin C, Russell L. Maternally inherited MAF variant associated with variable expression of Aymé-Gripp syndrome. Am J Med Genet A. 2019;179(11):2233–6. https://doi.org/10.1002/ajmg.a.61299.
Javadiyan S, Craig JE, Sharma S, Lower KM, Casey T, Haan E, Souzeau E, Burdon KP. Novel missense mutation in the bZIP transcription factor, MAF, associated with congenital cataract, developmental delay, seizures and hearing loss (Aymé-Gripp syndrome). BMC Med Genet. 2017;18(1):52. https://doi.org/10.1186/s12881-017-0414-7.
Sakai M, Imaki J, Yoshida K, Ogata A, Matsushima-Hibaya Y, Kuboki Y, et al. Rat maf related genes: specific expression in chondrocytes, lens and spinal cord. Oncogene. 1997;14(6):745–50. https://doi.org/10.1038/sj.onc.1200869.
Omoteyama K, Ikeda H, Imaki J, Sakai M. Activation of connective tissue growth factor gene by the c-Maf and Lc-Maf transcription factors. Biochem Biophys Res Commun. 2006;339(4):1089–97. https://doi.org/10.1016/j.bbrc.2005.11.119.
Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol. 2003;194(3):256–66. https://doi.org/10.1002/jcp.10206.
Bessant DA, Payne AM, Mitton KP, Wang QL, Swain PK, Plant C, et al. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat Genet. 1999;21(4):355–6. https://doi.org/10.1038/7678.
Tanahashi H, Kito K, Ito T, Yoshioka K. MafB protein stability is regulated by the JNK and ubiquitin-proteasome pathways. Archives of Biochemistry and Biophysics. 2010;494(1):94–100.
Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GC. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet. 2002;11(1):33–42. https://doi.org/10.1093/hmg/11.1.33.
Vanita V, Guo G, Singh D, Ott CE, Robinson PN. Differential effect of cataract-associated mutations in MAF on transactivation of MAF target genes. Mol Cell Biochem. 2014;396(1-2):137–45. https://doi.org/10.1007/s11010-014-2150-z.
Hansen L, Eiberg H, Rosenberg T. Novel MAF mutation in a family with congenital cataract-microcornea syndrome. Mol Vis. 2007;13:2019–22.
Hansen L, Mikkelsen A, Nurnberg P, Nurnberg G, Anjum I, Eiberg H, et al. Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Invest Ophthalmol Vis Sci. 2009;50(7):3291–303. https://doi.org/10.1167/iovs.08-3149.
Park JG, Tischfield MA, Nugent AA, Cheng L, Di Gioia SA, Chan WM, et al. Loss of MAFB function in humans and mice causes Duane syndrome, aberrant extraocular muscle innervation, and inner-ear defects. Am J Hum Genet. 2016;98(6):1220–7. https://doi.org/10.1016/j.ajhg.2016.03.023.
Sato Y, Tsukaguchi H, Morita H, Higasa K, Tran MTN, Hamada M, Usui T, Morito N, Horita S, Hayashi T, Takagi J, Yamaguchi I, Nguyen HT, Harada M, Inui K, Maruta Y, Inoue Y, Koiwa F, Sato H, et al. A mutation in transcription factor MAFB causes focal segmental glomerulosclerosis with Duane retraction syndrome. Kidney Int. 2018;94(2):396–407. https://doi.org/10.1016/j.kint.2018.02.025.
Kanai M, Jeon H, Ojima M, Nishino T, Usui T, Yadav MK, Kulathunga K, Morito N, Takahashi S, Hamada M. Phenotypic analysis of mice carrying human-type MAFB p.Leu239Pro mutation. Biochem Biophys Res Commun. 2020;523(2):452–7. https://doi.org/10.1016/j.bbrc.2019.12.033.
Nishikomori R, Kawai T, Toshiyuki K, Oda H, Yasumi T, Izawa K, Ohara O, Heike T Remarkable improvement of articular pain by biologics in a Multicentric carpotarsal osteolysis patient with a mutation of MAFB gene. Pediatric Rheumatology Online Journal 2015;13(Suppl 1): P152-P152.
Bhavani GS, Shah H, Shukla A, Girisha KM. Multicentric Osteolysis Nodulosis and Arthropathy. 2016 Jul 14 [Updated 2021 Sep 9]. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2022. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022.
Martignetti JA, Aqeel AA, Sewairi WA, Boumah CE, Kambouris M, Mayouf SA, Sheth KV, Eid WA, Dowling O, Harris J, Glucksman MJ, Bahabri S, Meyer BF, Desnick RJ. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet. 2001;28(3):261–5.
Zankl A, Packman L, Poznanski A, Bonafe L, Wang F, Shusterman Y, et al. Torg syndrome is caused by inactivating mutations in MMP2 and is allelic to NAO and Winchester syndrome. J Bone Miner Res. 2007;22(2):329–33.
Evans BR, Mosig RA, Lobl M, Martignetti CR, Camacho C, Grum-Tokars V, Glucksman MJ, Martignetti JA. Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am J Hum Genet. 2012;91(3):572–6.
Zhang Y, Ross AC. Retinoic acid and the transcription factor MafB act together and differentially to regulate aggrecan and matrix metalloproteinase gene expression in neonatal chondrocytes. J Cell Biochem. 2013;114(2):471–9.
Duerr S, Stremme S, Soeder S, Bau B, Aigner T. MMP-2/gelatinase A is a gene product of human adult articular chondrocytes and is increased in osteoarthritic cartilage. Clin Exp Rheumatol. 2004;22(5):603–8.
Mosig RA, Dowling O, DiFeo A, Ramirez M, Parker IC, Abe E, et al. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum Mol Genet. 2007;16(9):1113–23.
Lazarus S, Tseng HW, Lawrence F, Woodruff MA, Duncan EL, Pettit AR. Characterization of normal murine carpal bone development prompts re-evaluation of pathologic osteolysis as the cause of human carpal-tarsal osteolysis disorders. Am J Pathol. 2017;187(9):1923–34.
Zarei A, Gottesman G, Wenkert D, Nenninger A, Duan S, Bijanki VB, et al. 2021 Mutations causing multicentric carpotarsal osteolysis disrupt osteoblast and chondrocyte development. J Bone Miner Res 36 (Suppl 1). Available at https://www.asbmr.org/meetings/annualmeeting/AbstractDetail?aid=33899f08-0996-4e71-b9c6-c83246348a73. Accessed 3 Oct 2021.
Han Y, Shao W, Zhong D, Ma C, Wei X, Ahmed A, et al. Zebrafish mafbb mutants display osteoclast over-activation and bone deformity resembling osteolysis in MCTO patients. Biomolecules 2021;11(3).
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Nina Ma. Nina Ma and Michael Levine performed the literature search. All authors commented on previous versions of the manuscript. All authors read, critically revised, and approved the final manuscript.
Corresponding author
Ethics declarations
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
Nina Ma reports grants from Amgen and Ultragenyx, grants and personal fees from Ascendis Pharma, and others from Sophie’s Neighborhood and personal fees from UpToDate.
Steve Mumm reports grants from Shriners Children’s and others from Genomenon, Inc.
Satoru Takahashi reports grants from Sophie's Neighborhood.
Michael Levine reports he has been an investigator on a single-case study of the efficacy of denosumab for MCTO that was funded by Amgen, Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rare Bone Disease
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, N.S., Mumm, S., Takahashi, S. et al. Multicentric Carpotarsal Osteolysis: a Contemporary Perspective on the Unique Skeletal Phenotype. Curr Osteoporos Rep 21, 85–94 (2023). https://doi.org/10.1007/s11914-022-00762-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00762-7