Abstract
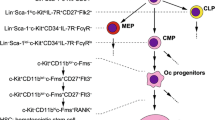
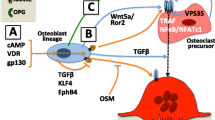
Understanding of osteoclast formation and activation has advanced considerably since the discovery of the RANKL/RANK/OPG system in the mid 1990s. Osteoblasts and stromal stem cells express receptor activator of NF-κB ligand (RANKL), which binds to its receptor, RANK, on the surface of osteoclasts and their precursors. This regulates the differentiation of precursors into multinucleated osteoclasts and osteoclast activation and survival both normally and in most pathologic conditions associated with increased bone resorption. Osteoprotegerin (OPG) is secreted by osteoblasts and osteogenic stromal stem cells and protects the skeleton from excessive bone resorption by binding to RANKL and preventing it from interacting with RANK. The RANKL/OPG ratio in bone marrow is thus an important determinant of bone mass in normal and disease states. RANKL/RANK signaling also regulates lymph node formation and mammary gland lactational hyperplasia in mice, and OPG protects large arteries of mice from medial calcification. This article reviews the roles of the RANKL/RANK/OPG system in bone and other tissues.
Similar content being viewed by others
References and Recommended Reading
Rodan GA, Martin TJ: Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int 1981, 33:349–351.
Martin TJ, Sims NA: Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 2005, 11:76–81.
Teitelbaum SL, Ross FP: Genetic regulation of osteoclast development and function. Nat Rev Genet 2003, 4:638–649.
Black DM, Greenspan SL, Ensrud KE, et al.: The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003, 349:1207–1215.
Ma YL, Bryant HU, Zeng Q, et al.: New bone formation with teriparatide [human parathyroid hormone-(1–34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology 2003, 144:2008–2015.
Lee SH, Rho J, Jeong D, et al.: v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 2006, 12:1403–1409.
Zhao C, Irie N, Takada Y, et al.: Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 2006, 4:111–121.
Simonet WS, Lacey DL, Dunstan CR, et al.: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997, 89:309–319.
Yasuda H, Shima N, Nakagawa N, et al.: Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1998, 139:1329–1337.
Yasuda H, Shima N, Nakagawa N, et al.: Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 1998, 95:3597–3602.
Lacey DL, Timms E, Tan HL, et al.: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165–176.
Anderson DM, Maraskovsky E, Billingsley WL, et al.: A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390:175–179.
Wong BR, Rho J, Arron J, et al.: TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 1997, 272:25190–25194.
American Society for Bone and Mineral Research President’s Committee on Nomenclature: Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. The American Society for Bone and Mineral Research President’s Committee on Nomenclature. J Bone Miner Res 2000, 15:2293–2296.
Ikeda T, Kasai M, Utsuyama M, Hirokawa K: Determination of three isoforms of the receptor activator of nuclear factor-κB ligand and their differential expression in bone and thymus. Endocrinology 2001, 142:1419–1426.
Lynch CC, Hikosaka A, Acuff HB, et al.: MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 2005, 7:485–496.
Schett G, Hayer S, Zwerina J, et al.: Mechanisms of disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol 2005, 1:47–54.
Takayanagi H, Kim S, Matsuo K, et al.: RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature 2002, 416:744–749.
Takayanagi H, Ogasawara K, Hida S, et al.: T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 2000, 408:600–605.
Takayanagi H: Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007, 7:292–304.
Feldmann M, Maini RN: Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 2003, 9:1245–1250.
Yao Z, Li P, Zhang Q, et al.: Tumor necrosis factor-α increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem 2006, 281:11846–11855.
Li P, Schwarz EM, O’Keefe RJ, et al.: Systemic tumor necrosis factor α mediates an increase in peripheral CD11b high osteoclast precursors in tumor necrosis factor α-transgenic mice. Arthritis Rheum 2004, 50:265–276.
Kollet O, Dar A, Shivtiel S, et al.: Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 2006, 12:657–664.
Kim NS, Kim HJ, Koo BK, et al.: Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol 2006, 26:1002–1013.
Fata JE, Kong Y-Y, Li J, et al.: The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 2000, 103:41–50.
Wada T, Nakashima T, Hiroshi N, Penninger JM: RANKLRANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 2006, 12:17–25.
Chen G, Sircar K, Aprikian A, et al.: Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer 2006, 107:289–298.
Hughes AE, Ralston SH, Marken J, et al.: Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis [letter]. Nat Genet 2000, 24:45–48.
Kapur RP, Yao Z, Iida Mh, et al.: Malignant autosomal recessive osteopetrosis caused by spontaneous mutation of murine Rank. J Bone Miner Res 2004, 19:1689–1697.
Whyte MP, Obrecht SE, Finnegan PM, et al.: Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med 2002, 347:175–184.
Cundy T, Hegde M, Naot D, et al.: A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet 2002, 11:2119–2127.
Hofbauer LC, Schoppet M: Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004, 292:490–495.
Boyce BF, Xing L, Chen D: Osteoprotegerin, the bone protector, is a surprising target for beta-catenin signaling. Cell Metab 2005, 2:344–345.
Bucay N, Sarosi I, Dunstan CR, et al.: Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998, 12:1260–1268.
Bennett BJ, Scatena M, Kirk EA, et al.: Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older apoE -/- mice. Arterioscler Thromb Vasc Biol 2006, 26:2117–2124.
Collin-Osdoby P: Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 2004, 95:1046–1057.
Rogers A, Eastell R: Circulating osteoprotegerin and receptor activator for nuclear factor κB ligand: clinical utility in metabolic bone disease assessment. J Clin Endocrinol Metab 2005, 90:6323–6331.
Lomaga MA, Yeh WC, Sarosi I, et al.: TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 1999, 13:1015–1024.
Naito A, Azuma S, Tanaka S, et al.: Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 1999, 4:353–362.
Kurihara N, Hiruma Y, Zhou H, et al.: Mutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget disease. J Clin Invest 2007, 117:133–142.
Takayanagi H, Kim S, Koga T, et al.: Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 2002, 3:889–901.
Phoon CK, Ji RP, Aristizabal O, et al.: Embryonic heart failure in NFATc1 -/- mice: novel mechanistic insights from in utero ultrasound biomicroscopy. Circ Res 2004, 95:92–99.
Yao Z, Matsuo K, Nishimura R, et al.: c-Fos/NFAT1-or 2-mediated osteoclastogenesis requires NF-κB p50/p52 expression [abstract]. J Bone Miner Res 2005, 20(suppl 1):S145.
Asagiri M, Sato K, Usami T, et al.: Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 2005, 202:1261–1269.
Nakashima K, Zhou X, Kunkel G, et al.: The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108:17–29.
Koga T, Matsui Y, Asagiri M, et al.: NFAT and osterix cooperatively regulate bone formation. Nat Med 2005, 11:880–885.
Thiebaud D, Krieg MA, Gillard-Berguer D, et al.: Cyclosporine induces high bone turnover and may contribute to bone loss after heart transplantation. Eur J Clin Invest 1996, 26:549–555.
Koga T, Inui M, Inoue K, et al.: Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 2004, 428:758–763.
Takayanagi H: Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 2005, 83:170–179.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyce, B.F., Xing, L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep 5, 98–104 (2007). https://doi.org/10.1007/s11914-007-0024-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-007-0024-y