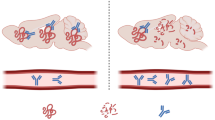
Abstract
Purpose of Review
This review summarizes previous and ongoing neuroprotection trials in multiple system atrophy (MSA), a rare and fatal neurodegenerative disease characterized by parkinsonism, cerebellar, and autonomic dysfunction. It also describes the preclinical therapeutic pipeline and provides some considerations relevant to successfully conducting clinical trials in MSA, i.e., diagnosis, endpoints, and trial design.
Recent Findings
Over 30 compounds have been tested in clinical trials in MSA. While this illustrates a strong treatment pipeline, only two have reached their primary endpoint. Ongoing clinical trials primarily focus on targeting α-synuclein, the neuropathological hallmark of MSA being α-synuclein-bearing glial cytoplasmic inclusions.
Summary
The mostly negative trial outcomes highlight the importance of better understanding underlying disease mechanisms and improving preclinical models. Together with efforts to refine clinical measurement tools, innovative statistical methods, and developments in biomarker research, this will enhance the design of future neuroprotection trials in MSA and the likelihood of positive outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Poewe W, Stankovic I, Halliday G, Meissner WG, Wenning GK, Pellecchia MT, Seppi K, Palma J-A, Kaufmann H. Multiple system atrophy. Nat Rev Dis Primers. 2022;8:1–21.
Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM, Parisi JE. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138:2293–309.
Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. The Lancet Neurology. 2013;12:264–74.
Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM-Y. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in non-transgenic mice. Science. 2012;338:949–53.
Wong YC, Krainc D. α-Synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23:1–13.
Inoue M, Yagishita S, Ryo M, Hasegawa K, Amano N, Matsushita M. The distribution and dynamic density of oligodendroglial cytoplasmic inclusions (GCIs) in multiple system atrophy: a correlation between the density of GCIs and the degree of involvement of striatonigral and olivopontocerebellar systems. Acta Neuropathol. 1997;93:585–91.
Ozawa T, Paviour D, Quinn NP, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127:2657–71.
Peng C, Gathagan RJ, Covell DJ, et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature. 2018;557:558–63.
• Shahnawaz M, Mukherjee A, Pritzkow S, et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature. 2020;578:273–7. This article explores the accuracy of a seed amplification assay to detect different patterns of alpha-synuclein aggregation between MSA and PD patients. They combined different methods to compare these patterns and observed distinct conformational strains of alpha-synuclein between MSA and PD
• Schweighauser M, Shi Y, Tarutani A, et al. Structures of α-synuclein filaments from multiple system atrophy. Nature. 2020;585:464–9. Using cryo-electron microscopy, the authors observed different structures of alpha-synuclein filaments in MSA and between MSA and DLB patients. This suggests that different alpha-synuclein strains could be related to distinct synucleinopathies
•• Wenning GK, Stankovic I, Vignatelli L, et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Movement Disorders. 2022;37:1131–48. This revision of diagnostic criteria of MSA aims to improve diagnosis accuracy at earlier stages of the disease and describes a new category of clinically established MSA that requires the presence of brain imaging features
Miki Y, Foti SC, Asi YT, Tsushima E, Quinn N, Ling H, Holton JL. Improving diagnostic accuracy of multiple system atrophy: a clinicopathological study. Brain. 2019;142:2813–27.
Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW. When DLB, PD, and PSP masquerade as MSA. Neurology. 2015;85:404–12.
Osaki Y, Ben-Shlomo Y, Lees AJ, Wenning GK, Quinn NP. A validation exercise on the new consensus criteria for multiple system atrophy. Mov Disord. 2009;24:2272–6.
• Virameteekul S, Revesz T, Jaunmuktane Z, Warner TT, De Pablo-Fernández E. Pathological validation of the MDS criteria for the diagnosis of multiple system atrophy. Movement Disorders. 2023;38:444–52. This article provides evidence of enhanced diagnostic performances of the new diagnostic criteria of MSA and excellent accuracy of the new category of clinically established MSA, even at the early stages of the disease
Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord. 2004;19:1391–402.
•• Krismer F, Palma J-A, Calandra-Buonaura G, et al. The unified multiple system atrophy rating scale: status, critique, and recommendations. Movement Disorders. 2022;37:2336–41. This review article describes the main limitation of the UMSARS and provides a roadmap to a revised version of the scale. The authors announced the creation of a task force of experts and the preparatory steps for an exhaustive documentation to provide a more comprehensive and patient-centered scale
Krismer F, Seppi K, Jönsson L, et al. Sensitivity to change and patient-centricity of the unified multiple system atrophy rating scale items: a data-driven analysis. Movement Disorders. 2022;37:1425–31.
Potashman M, Brady L, Durham S, et al (2023) Psychometric validation of a modified united multiple system atrophy rating scale (S43.002). In: Wednesday, April 26. Lippincott Williams & Wilkins, p 1958
Potashman M, Huang I, Durham S, et al. Patient concept elicitation interviews: insights into multiple system atrophy (MSA) patient experiences and relevance of a modified united multiple system atrophy rating scale (P8-9.003). In: Tuesday, April 25. Lippincott Williams & Wilkins; 2023. p. 2057.
Palma J-A, Vernetti PM, Perez MA, et al. Limitations of the Unified Multiple System Atrophy Rating Scale as outcome measure for clinical trials and a roadmap for improvement. Clin Auton Res. 2021;31:157–64.
Foubert-Samier A, Pavy-Le Traon A, Saulnier T, Le-Goff M, Fabbri M, Helmer C, Rascol O, Proust-Lima C, Meissner WG. An item response theory analysis of the unified multiple system atrophy rating scale. Parkinsonism & Related Disorders. 2022;94:40–4.
Foubert-Samier A, Pavy-Le Traon A, Guillet F, Le-Goff M, Helmer C, Tison F, Rascol O, Proust-Lima C, Meissner WG. Disease progression and prognostic factors in multiple system atrophy: a prospective cohort study. Neurobiol Dis. 2020;139:104813.
Saulnier T, Philipps V, Meissner WG, Rascol O, Pavy-Le Traon A, Foubert-Samier A, Proust-Lima C. Joint models for the longitudinal analysis of measurement scales in the presence of informative dropout. Methods. 2022;203:142–51.
Péran P, Barbagallo G, Nemmi F, Sierra M, Galitzky M, Traon AP-L, Payoux P, Meissner WG, Rascol O. MRI supervised and unsupervised classification of Parkinson’s disease and multiple system atrophy. Mov Disord. 2018;33:600–8.
Chougar L, Faouzi J, Pyatigorskaya N, et al. Automated categorization of Parkinsonian syndromes using magnetic resonance imaging in a clinical setting. Movement Disorders. 2021;36:460–70.
• Smith R, Capotosti F, Schain M, et al. The α-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases. Nat Commun. 2023;14:6750. This article shows a promising perspective for the use of an alpha-synuclein PET tracer to enhance diagnostic performances. The authors presented a specific biding to strategic brain regions related to MSA pathology and the ability of the tracer to distinguish between MSA patients and healthy controls or other synucleinopathies
• Poggiolini I, Gupta V, Lawton M, et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain. 2022;145:584–95. In this article, the authors explored the ability of seed amplification assay to distinguish between synucleinopathies and predict disease conversion of REM-sleep behavior disorder. This highlights the potential use of this method to detect synucleinopathy at prodromal stages
Rossi M, Candelise N, Baiardi S, et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020;140:49–62.
Chelban V, Nikram E, Perez-Soriano A, et al. Neurofilament light levels predict clinical progression and death in multiple system atrophy. Brain. 2022;145:4398–408.
Singer W, Schmeichel AM, Shahnawaz M, et al. Alpha-synuclein oligomers and neurofilament light chain in spinal fluid differentiate multiple system atrophy from Lewy body synucleinopathies. Ann Neurol. 2020;88:503–12.
Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, et al. Targeting huntingtin expression in patients with Huntington’s disease. New England Journal of Medicine. 2019;380:2307–16.
Wave Life Sciences Provides Update on Phase 1b/2a PRECISION-HD Trials - Wave Life Sciences. https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-provides-update-phase-1b2a-precision-hd. Accessed 17 May 2023
Alarcón-Arís D, Recasens A, Galofré M, et al. Selective α-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for Parkinson’s disease. Mol Ther. 2018;26:550–67.
Uehara T, Choong C-J, Nakamori M, et al. Amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotides targeting α-synuclein as a novel therapy for Parkinson’s disease. Sci Rep. 2019;9:7567.
Miquel-Rio L, Alarcón-Arís D, Torres-López M, et al. Human α-synuclein overexpression in mouse serotonin neurons triggers a depressive-like phenotype. Rescue by oligonucleotide therapy. Transl Psychiatry. 2022;12:79.
Kallab M, Herrera-Vaquero M, Johannesson M, Eriksson F, Sigvardson J, Poewe W, Wenning GK, Nordström E, Stefanova N. Region-specific effects of immunotherapy with antibodies targeting α-synuclein in a transgenic model of synucleinopathy. Front Neurosci. 2018;12:452.
Schofield DJ, Irving L, Calo L, et al. Preclinical development of a high affinity α-synuclein antibody, MEDI1341, that can enter the brain, sequester extracellular α-synuclein and attenuate α-synuclein spreading in vivo. Neurobiol Dis. 2019;132:104582.
Knecht L, Folke J, Dodel R, Ross JA, Albus A. Alpha-synuclein immunization strategies for synucleinopathies in clinical studies: a biological perspective. Neurotherapeutics. 2022;19:1489–502.
Schneeberger A, Mandler M, Mattner F, Schmidt W. AFFITOME® technology in neurodegenerative diseases: the doubling advantage. Hum Vaccin. 2010;6:948–52.
Levin J, Maaß S, Schuberth M, et al. Safety and efficacy of epigallocatechin gallate in multiple system atrophy (PROMESA): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019;18:724–35.
Finkelstein DI, Billings JL, Adlard PA, et al. The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson’s disease. Acta Neuropathol Commun. 2017;5:53.
Levin J, Maaß S, Schuberth M, et al. The PROMESA-protocol: progression rate of multiple system atrophy under EGCG supplementation as anti-aggregation-approach. J Neural Transm. 2016;123:439–45.
Stamler D, Bradbury M, Wong C, Offman E. A phase 1 study of PBT434, a novel small molecule inhibitor of α-synuclein aggregation, in adult and older adult volunteers (4871). Neurology. 2020;94:4871
Devos D, Labreuche J, Rascol O, et al. Trial of deferiprone in Parkinson’s disease. N Engl J Med. 2022;387:2045–55.
Herrera-Vaquero M, Bouquio D, Kallab M, et al. The molecular tweezer CLR01 reduces aggregated, pathologic, and seeding-competent α-synuclein in experimental multiple system atrophy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:165513.
Bengoa-Vergniory N, Faggiani E, Ramos-Gonzalez P, et al. CLR01 protects dopaminergic neurons in vitro and in mouse models of Parkinson’s disease. Nat Commun. 2020;11:4885.
Bassil F, Fernagut P-O, Bezard E, Pruvost A, Leste-Lasserre T, Hoang QQ, Ringe D, Petsko GA, Meissner WG. Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy. Proc Natl Acad Sci U S A. 2016;113:9593–8.
Arotcarena M-L, Bourdenx M, Dutheil N, et al. Transcription factor EB overexpression prevents neurodegeneration in experimental synucleinopathies. JCI Insight. 2019;4(e129719):129719.
Mahul-Mellier A-L, Fauvet B, Gysbers A, et al. c-Abl phosphorylates α-synuclein and regulates its degradation: implication for α-synuclein clearance and contribution to the pathogenesis of Parkinson’s disease. Hum Mol Genet. 2014;23:2858–79.
Lopez-Cuina M, Guerin PA, Canron M-H, Delamarre A, Dehay B, Bezard E, Meissner WG, Fernagut P-O. Nilotinib fails to prevent synucleinopathy and cell loss in a mouse model of multiple system atrophy. Mov Disord. 2020;35:1163–72.
Inhibikase therapeutics announces FDA has lifted the full clinical hold on IkT-148009 in multiple system atrophy. In: Inhibikase Therapeutics, Inc. 2023 https://www.inhibikase.com/news/press-releases/detail/77/inhibikase-therapeutics-announces-fda-has-lifted-the-full. Accessed 3 Nov 2023
Bassil F, Canron M-H, Vital A, Bezard E, Li Y, Greig NH, Gulyani S, Kapogiannis D, Fernagut P-O, Meissner WG. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. 2017;140:1420–36.
Dodel R, Spottke A, Gerhard A, et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov Disord. 2010;25:97–107.
Vidal-Martinez G, Segura-Ulate I, Yang B, Diaz-Pacheco V, Barragan JA, De-Leon Esquivel J, Chaparro SA, Vargas-Medrano J, Perez RG. FTY720-Mitoxy reduces synucleinopathy and neuroinflammation, restores behavior and mitochondria function, and increases GDNF expression in Multiple System Atrophy mouse models. Exp Neurol. 2020;325:113120.
Novak P, Williams A, Ravin P, Zurkiya O, Abduljalil A, Novak V. Treatment of multiple system atrophy using intravenous immunoglobulin. BMC Neurol. 2012;12:131.
Mitsui J, Matsukawa T, Uemura Y, et al. High-dose ubiquinol supplementation in multiple-system atrophy: a multicentre, randomised, double-blinded, placebo-controlled phase 2 trial. eClinicalMedicine. 2023; https://doi.org/10.1016/j.eclinm.2023.101920.
Holmberg B, Johansson J-O, Poewe W, et al. Safety and tolerability of growth hormone therapy in multiple system atrophy: a double-blind, placebo-controlled study. Movement Disorders. 2007;22:1138–44.
Mullen JA, Savage AB, Minkwitz MC, Jucaite A, Cselényi Z, Johnström P, Posener J, Kugler A, Wenning G, Kaufmann H, Barone P, Meissner W, Carson R, Kreisl WC, Rabiner EA, Farde L, Poewe W, on behalf of the MSA Study Group. Safety, biomarker effects, and efficacy of the myeloperoxidase inhibitor AZD3241 in patients with multiple system atrophy: a 12-week randomized multicenter PET study (P.6016). Abstracts of the 6th international multiple system atrophy congress. Clin Auton Res 2018;28:137–60.
Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19:673–94.
Cole TA, Zhao H, Collier TJ, et al. α-Synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson’s disease. JCI Insight. 2021;6(e135633):135633.
Boutros SW, Raber J, Unni VK. Effects of alpha-synuclein targeted antisense oligonucleotides on Lewy body-like pathology and behavioral disturbances induced by injections of pre-formed fibrils in the mouse motor cortex. J Parkinsons Dis. 2021;11:1091–115.
Yang J, Luo S, Zhang J, Yu T, Fu Z, Zheng Y, Xu X, Liu C, Fan M, Zhang Z. Exosome-mediated delivery of antisense oligonucleotides targeting α-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol Dis. 2021;148:105218.
Pavia-Collado R, Cóppola-Segovia V, Miquel-Rio L, et al. Intracerebral administration of a ligand-ASO conjugate selectively reduces α-synuclein accumulation in Monoamine Neurons of Double mutant human A30P*A53T*α-synuclein transgenic mice. Int J Mol Sci. 2021;22:2939.
Abeliovich A, Schmitz Y, Fariñas I, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–52.
Valera E, Spencer B, Fields JA, Trinh I, Adame A, Mante M, Rockenstein E, Desplats P, Masliah E. Combination of alpha-synuclein immunotherapy with anti-inflammatory treatment in a transgenic mouse model of multiple system atrophy. Acta Neuropathol Commun. 2017;5:2.
Shering C, Pomfret M, Kubiak R, et al. Reducing α-synuclein in human CSF; an evaluation of safety, tolerability, pharmacokinetics and pharmacodynamics of MEDI1341, an α-synuclein-specific antibody, in healthy volunteers and Parkinson’s disease patients (P1-11.007). Neurology. 2023; https://doi.org/10.1212/WNL.0000000000202579.
Fjord-Larsen L, Thougaard A, Wegener KM, Christiansen J, Larsen F, Schrøder-Hansen LM, Kaarde M, Ditlevsen DK. Nonclinical safety evaluation, pharmacokinetics, and target engagement of Lu AF82422, a monoclonal IgG1 antibody against alpha-synuclein in development for treatment of synucleinopathies. MAbs. 13:1994690.
Buur L, Wiedemann J, Larsen F, Ben Alaya-Fourati F, Kallunki P, Ditlevsen D, Sørensen M, Meulien D. The anti-alpha-synuclein antibody Lu AF82422 was safe and well tolerated in a FIH-SAD study in healthy subjects and patients with PD [abstract]. Mov Disord. 2022;37(suppl 2).
Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–62.
Mandler M, Valera E, Rockenstein E, et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127:861–79.
Mandler M, Valera E, Rockenstein E, et al. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener. 2015;10:10.
Mandler M, Rockenstein E, Overk C, et al. Effects of single and combined immunotherapy approach targeting amyloid β protein and α-synuclein in a dementia with Lewy bodies-like model. Alzheimers Dement. 2019;15:1133–48.
Volc D, Poewe W, Kutzelnigg A, et al. Safety and immunogenicity of the α-synuclein active immunotherapeutic PD01A in patients with Parkinson’s disease: a randomised, single-blinded, phase 1 trial. Lancet Neurol. 2020;19:591–600.
Poewe W, Volc D, Seppi K, et al. Safety and tolerability of active immunotherapy targeting α-synuclein with PD03A in patients with early Parkinson’s disease: a randomized, placebo-controlled, phase 1 study. J Parkinsons Dis. 2021;11:1079–89.
Meissner WG, Traon AP-L, Foubert-Samier A, et al. A phase 1 randomized trial of specific active α-synuclein immunotherapies PD01A and PD03A in multiple system atrophy. Movement Disorders. 2020;35:1957–65.
Yu HJ, Thijssen E, van Brummelen E, van der Plas JL, Radanovic I, Moerland M, Hsieh E, Groeneveld GJ, Dodart J-C. A randomized first-in-human study with UB-312, a UBITh® α-synuclein peptide vaccine. Mov Disord. 2022;37:1416–24.
Vaxxinity completes enrollment in part B of UB-312 phase 1 clinical trial for Parkinson’s disease | Vaxxinity. https://ir.vaxxinity.com/news-releases/news-release-details/vaxxinity-completes-enrollment-part-b-ub-312-phase-1-clinical/. Accessed 12 Apr 2023
Ubhi K, Rockenstein E, Mante M, Patrick C, Adame A, Thukral M, Shults C, Masliah E. Rifampicin reduces alpha-synuclein in a transgenic mouse model of multiple system atrophy. Neuroreport. 2008;19:1271–6.
Low PA, Robertson D, Gilman S, et al. Efficacy and safety of rifampicin for multiple system atrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:268–75.
Finkelstein DI, Shukla JJ, Cherny RA, Billings JL, Saleh E, Stefanova N, Barnham KJ, Adlard PA. The compound ATH434 prevents alpha-synuclein toxicity in a murine model of multiple system atrophy. J Parkinsons Dis. 2022;12:105–15.
Heras-Garvin A, Refolo V, Schmidt C, Malfertheiner K, Wenning GK, Bradbury M, Stamler D, Stefanova N. ATH434 reduces α-synuclein-related neurodegeneration in a murine model of multiple system atrophy. Movement Disorders. 2021;36:2605–14.
Heras-Garvin A, Weckbecker D, Ryazanov S, Leonov A, Griesinger C, Giese A, Wenning GK, Stefanova N. Anle138b modulates α-synuclein oligomerization and prevents motor decline and neurodegeneration in a mouse model of multiple system atrophy. Movement Disorders. 2019;34:255–63.
Levin J, Schmidt F, Boehm C, Prix C, Bötzel K, Ryazanov S, Leonov A, Griesinger C, Giese A. The oligomer modulator anle138b inhibits disease progression in a Parkinson mouse model even with treatment started after disease onset. Acta Neuropathol. 2014;127:779–80.
Levin J, Sing N, Melbourne S, et al. Safety, tolerability and pharmacokinetics of the oligomer modulator anle138b with exposure levels sufficient for therapeutic efficacy in a murine Parkinson model: a randomised, double-blind, placebo-controlled phase 1a trial. eBioMedicine. 2022; https://doi.org/10.1016/j.ebiom.2022.104021.
Levin J, Singh N, Melbourne S, Morgan A, Carroll C, Fietzek U, Ryazanov S, Leonov A, Griesinger CH, Schmidt F, Weckbecker D, Prager K, Matthias T, Giese A. Anle138b-P1-02: a randomised, double-blinded, placebo-controlled phase 1b study to investigate safety, tolerability, pharmacokinetics and pharmacodynamics of the oligomer modulator anle138b in Parkinson’s disease [abstract]. Mov Disord. 2023;38(suppl 1).
Lee H-J, Khoshaghideh F, Patel S, Lee S-J. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–96.
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5.
Palma J-A, Martinez J, Millar Vernetti P, et al. mTOR inhibition with sirolimus in multiple system atrophy: a randomized, double-blind, placebo-controlled futility trial and 1-year biomarker longitudinal analysis. Mov Disord. 2022;37:778–89.
Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK, Stefanova N. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy. Mol Neurodegener. 2017;12:52.
Valera E, Spencer B, Mott J, et al. MicroRNA-101 modulates autophagy and oligodendroglial alpha-synuclein accumulation in multiple system atrophy. Front Mol Neurosci. 2017;10:329.
Spencer B, Valera E, Rockenstein E, Trejo-Morales M, Adame A, Masliah E. A brain-targeted, modified neurosin (kallikrein-6) reduces α-synuclein accumulation in a mouse model of multiple system atrophy. Mol Neurodegener. 2015;10:48.
Kiely AP, Miners JS, Courtney R, Strand C, Love S, Holton JL. Exploring the putative role of kallikrein-6, calpain-1 and cathepsin-D in the proteolytic degradation of α-synuclein in multiple system atrophy. Neuropathol Appl Neurobiol. 2019;45:347–60.
Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: The NNIPPS Study. Brain. 2009;132:156–71.
Friess E, Kuempfel T, Modell S, Winkelmann J, Holsboer F, Ising M, Trenkwalder C. Paroxetine treatment improves motor symptoms in patients with multiple system atrophy. Parkinsonism Relat Disord. 2006;12:432–7.
Rascol O, Cochen de Cock V, Pavy-Le Traon A, et al. Fluoxetine for the symptomatic treatment of multiple system atrophy: the MSA-FLUO trial. Mov Disord. 2021;36:1704–11.
Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, Rockenstein E, May V, Winkler J, Masliah E. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy. Exp Neurol. 2012;234:405–16.
Meyer M, Lamare F, Asselineau J, et al. Brain 5-HT1A receptor binding in multiple system atrophy: an [18 F]-MPPF PET study. Mov Disord. 2021;36:246–51.
Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E. Antidepressants reduce neuroinflammatory responses and astroglial alpha-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia. 2014;62:317–37.
Lee PH, Lee JE, Kim H-S, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40.
Singer W, Dietz AB, Zeller AD, et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology. 2019;93:e77–87.
Stefanova N, Poewe W, Wenning GK. Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol. 2008;210:421–7.
Poewe W, Seppi K, Fitzer-Attas CJ, et al. Efficacy of rasagiline in patients with the parkinsonian variant of multiple system atrophy: a randomised, placebo-controlled trial. Lancet Neurol. 2015;14:145–52.
Park HS, Song YS, Moon BS, Yoo S-E, Lee JM, Chung Y-T, Kim E, Lee BC, Kim SE. Neurorestorative effects of a novel fas-associated factor 1 inhibitor in the MPTP model: an [18F]FE-PE2I positron emission tomography analysis study. Front Pharmacol. 2020;11:953.
Shin W, Lim KS, Kim M-K, et al. A first-in-human study to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of KM-819 (FAS-associated factor 1 inhibitor), a drug for Parkinson’s disease, in healthy volunteers. Drug Des Devel Ther. 2019;13:1011–22.
Tardiff DF, Lucas M, Wrona I, Chang B, Chung CY, Le Bourdonnec B, Rhodes KJ, Scannevin RH. Non-clinical pharmacology of YTX-7739: a clinical stage stearoyl-CoA desaturase inhibitor being developed for Parkinson’s disease. Mol Neurobiol. 2022;59:2171–89.
Vincent BM, Tardiff DF, Piotrowski JS, et al. Inhibiting stearoyl-CoA desaturase ameliorates α-synuclein cytotoxicity. Cell Rep. 2018;25:2742–2754.e31.
Inc YT (2021) Yumanity Therapeutics’ YTX-7739 achieved target engagement at doses that were generally well tolerated in a phase 1a multiple ascending dose study in healthy volunteers. In: GlobeNewswire News Room. https://www.globenewswire.com/news-release/2021/04/22/2214878/0/en/Yumanity-Therapeutics-YTX-7739-Achieved-Target-Engagement-at-Doses-That-Were-Generally-Well-Tolerated-in-a-Phase-1a-Multiple-Ascending-Dose-Study-in-Healthy-Volunteers.html. Accessed 2 Nov 2023
Vargas-Medrano J, Segura-Ulate I, Yang B, Chinnasamy R, Arterburn JB, Perez RG. FTY720-Mitoxy reduces toxicity associated with MSA-like α-synuclein and oxidative stress by increasing trophic factor expression and myelin protein in OLN-93 oligodendroglia cell cultures. Neuropharmacology. 2019;158:107701.
Sturm E, Fellner L, Krismer F, Poewe W, Wenning GK, Stefanova N. Neuroprotection by epigenetic modulation in a transgenic model of multiple system atrophy. Neurotherapeutics. 2016;13:871–9.
Ettle B, Kerman BE, Valera E, et al. α-Synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy. Acta Neuropathol. 2016;132:59–75.
Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369:233–44.
Barca E, Kleiner G, Tang G, et al. Decreased coenzyme Q10 levels in multiple system atrophy cerebellum. Journal of Neuropathology & Experimental Neurology. 2016;75:663–72.
Compta Y, Giraldo DM, Muñoz E, et al. Cerebrospinal fluid levels of coenzyme Q10 are reduced in multiple system atrophy. Parkinsonism & Related Disorders. 2018;46:16–23.
Schottlaender LV, Bettencourt C, Kiely AP, Chalasani A, Neergheen V, Holton JL, Hargreaves I, Houlden H. Coenzyme Q10 levels are decreased in the cerebellum of multiple-system atrophy patients. PLOS ONE. 2016;11:e0149557.
Mitsui J, Matsukawa T, Tanaka M, et al. Randomized, double-blind, placebo-controlled phase 1 study to evaluate the safety and pharmacokinetics of high doses of ubiquinol in healthy adults. Neurology and Clinical Neuroscience. 2022;10:14–24.
Krismer F, Seppi K, Wenning GK, Abler V, Papapetropoulos S, Poewe W. Minimally clinically important decline in the parkinsonian variant of multiple system atrophy. Mov Disord. 2016;31:1577–81.
Bassil F, Fernagut P-O, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1–18.
Lopez-Cuina M, Guérin P, Dutheil N, Martin C, Lasserre TL, Fernagut P-O, Meissner WG, Bezard E. GRK2-targeted knockdown as therapy for multiple system atrophy. Mov Disord. 2023; https://doi.org/10.1002/mds.29422.
Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22:2196–203.
Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012;21:393–404.
Kaindlstorfer C, Sommer P, Georgievska B, Mather RJ, Kugler AR, Poewe W, Wenning GK, Stefanova N. Failure of neuroprotection despite microglial suppression by delayed-start myeloperoxidase inhibition in a model of advanced multiple system atrophy: clinical implications. Neurotox Res. 2015;28:185–94.
Jucaite A, Svenningsson P, Rinne JO, et al. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson’s disease. Brain. 2015;138:2687–700.
Fukae J, Fujioka S, Yanamoto S, Mori A, Nomi T, Hatano T, Fukuhara K, Ouma S, Hattori N, Tsuboi Y. Serum uric acid level is linked to the disease progression rate in male patients with multiple system atrophy. Clinical Neurology and Neurosurgery. 2017;158:15–9.
Yoo HS, Chung SJ, Lee YH, Ye BS, Sohn YH, Kwon H, Lee PH. Urate is closely linked to white matter integrity in multiple system atrophy. Ann Clin Transl Neurol. 2020;7:1029–39.
Jung Lee J, Han Yoon J, Jin Kim S, et al. Inosine 5’-Monophosphate to Raise Serum Uric Acid Level in Multiple System Atrophy (IMPROVE-MSA study). Clinical Pharmacology & Therapeutics. 2021;109:1274–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
M.F. received Grants from MSA Coalition, HORIZON 2022, Honoraria to speak from BIAL, AbbVie, Orkyn, Elivie, LvL médical and consultancies from Bial, Convatec and LvL médicale.
A.F.S. received honoraria from Aguettant Laboratory and Sanofi, grants from the French Rare Disease Foundation, from the French regional health agency (Agence Régionale de Santé de nouvelle Aquitaine), and from France Parkinson association.
W.G.M. reports personal fees from Elsevier, from Biohaven, Lundbeck, Roche, Servier, Alterity, Inhibikase, Teva and Takeda, outside the submitted work; and 1. International Parkinson and movement disorder society (Treasurer and Officer) 2. MSA Coalition (Member research steering committee) 3. Clément Fayat Foundation (Board of directors).
A.P.L. reports honoraria from Biohaven, HAC Pharma and speaker fees from Alnylam, outside the submitted work.
O.R. is advising following compagnies AbbVie, Acorda, Aguettant, Alkahest, AlzProtect, Apopharma, Astrazeneca, Bial, Biogen, Britannia, Buckwang, Cerevel, Clevexel, Contera, GE Healthcare, Handltherapeutic, Ionis, Irlab, Jazz, Kyowa, LGD Nuvamid, Lundbeck, Merck, Merz, MundiPharma, Neuralight, Neuratris, Neuroderm, Novartis, ONO Pharma, Orion Pharma, Parexel, PD Neurotechnology, Pfizer, Polycaps, Prexton, Roche Therapeutics, Sanofi, Scienture, Servier, Sombiotech, Sunovion, Supernus, Synagile, Thelonius Mind, Takeda, Théranexus, Teva, Tools4patient, UCB, Vision 2 voice, Zambon.
C.P.L., D.B., F.S., P.O.F., and T.S. have nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bendetowicz, D., Fabbri, M., Sirna, F. et al. Recent Advances in Clinical Trials in Multiple System Atrophy. Curr Neurol Neurosci Rep 24, 95–112 (2024). https://doi.org/10.1007/s11910-024-01335-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-024-01335-0