Abstract
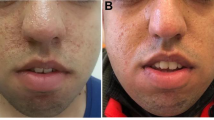
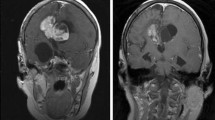
Neurofibromatosis type 1 (NF1) and tuberous sclerosis complex (TSC) are autosomal-dominant genetic disorders that result from dysregulation of the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway. NF1 is caused by mutations in the NF1 gene on chromosome 17q11.2. Its protein product, neurofibromin, functions as a tumor suppressor and ultimately produces constitutive upregulation of mTOR. TSC is caused by mutations in either the TSC1 (chromosome 9q34) or TSC2 (chromosome 16p.13.3) genes. Their protein products, hamartin and tuberin, respectively, form a dimer that acts via the GAP protein Rheb (Ras homolog enhanced in brain) to directly inhibit mTOR, again resulting in upregulation. Specific inhibitors of mTOR are in clinical use, including sirolimus, everolimus, temsirolimus, and deforolimus. Everolimus has been shown to reduce the volume and appearance of subependymal giant cell astrocytomas (SEGA), facial angiofibromas, and renal angiomyolipomas associated with TSC, with a recent FDA approval for SEGA not suitable for surgical resection. This article reviews the use of mTOR inhibitors in these diseases, which have the potential to be a disease-modifying therapy in these and other conditions.

Similar content being viewed by others
References
Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78.
Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, et al. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leukemia. 2000;14(12):2223–33.
Rechnitzer C. Increased survival of children with solid tumours: how did we get there and how to keep the success going? Canc Imag. 2011;11:865–9.
Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute, Bethesda, MD; 2010.
Stoddart A, Fleming HE, Paige CJ. The role of the preBCR, the interleukin-7 receptor, and homotypic interactions during B-cell development. Immunol Rev. 2000;175:47–58.
Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102(24):8573–8.
Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65(7):2755–60.
Perry B, Banyard J, McLaughlin ER, Watnick R, Sohn A, Brindley DN, et al. AKT1 overexpression in endothelial cells leads to the development of cutaneous vascular malformations in vivo. Arch Dermatol. 2007;143(4):504–6.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501.
Chiarini F, Fala F, Tazzari PL, Ricci F, Astolfi A, Pession A, et al. Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as a new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res. 2009;69(8):3520–8.
Houghton PJ, Huang S. mTOR as a target for cancer therapy. Curr Top Microbiol Immunol. 2004;279:339–59.
Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–48.
Bhaskar PT, Hay N. The two TORCs and Akt. Developmental Cell. 2007;12(4):487–502.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101.
Korf BR. Malignancy in neurofibromatosis type 1. Oncologist. 2000;5(6):477–85.
Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, Van RN, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15(6):541–55.
US Department of Health and Human Services, National Institutes of Health. Neurofibromatosis. NIH Consens Statement Online 1987 Jul 13–15;6(12):1–19.
Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89(1):31–7.
Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: The Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23:8422–30.
Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis. J Mol Genet. 2002;39:311–4.
Needle MN, Cnaan A, Dattilo J, Chatten J, Phillips PC, Shochat S, et al. Prognostic signs in the surgical management of plexiform neurofibroma: the Children's Hospital of Philadelphia experience, 1974–1994. J Pediatr. 1997;131(5):678–82.
Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. A clinical and population study in south-east Wales. Brain. 1988;111(Pt 6):1355–81.
Ricardi VM, Eichner JE. Neurofibromitosis: phenotype, natural history and pathogenesis. Baltimore: Johns Hopkins University Press; 1986.
Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68(5):1110–8.
Solomon J, Warren K, Dombi E, et al. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imag Graph. 2004;28:257–65.
Gupta A, Cohen BH, Ruggieri P, Packer RJ, Phillips PC. Phase I study of thalidomide for the treatment of plexiform neurofibroma in neurofibromatosis 1. Neurology. 2003;60(1):130–2.
Widemann BC, Salzer WL, Arceci RJ, Blaney SM, Fox E, End D, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24(3):507–16.
Babovic-Vuksanovic D, Widemann BC, Dombi E, Gillespie A, Wolters PL, Toledo-Tamula MA, et al. Phase I trial of pirfenidone in children with neurofibromatosis 1 and plexiform neurofibromas. Pediatr Neurol. 2007;36(5):293–300.
Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345–56.
Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–68.
Franz DN, Bissler JJ, McCormack FX. Tuberous sclerosis complex: neurological, renal and pulmonary manifestations. Neuropediatrics. 2010;41(5):199–208.
Baskin Jr HJ. The pathogenesis and imaging of the tuberous sclerosis complex. Pediatr Radiol. 2008;38(9):936–52.
Sancak O, Nellist M, Goedbloed M, Elfferich P, Wouters C, Maat-Kievit A, et al. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype: phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur J Hum Genet. 2005;13(6):731–41.
Anlauf M, Garbrecht N, Bauersfeld J, Schmitt A, Henopp T, Komminoth P, et al. Hereditary neuroendocrine tumors of the gastroenteropancreatic system. Virchows Arch. 2007;451 Suppl 1:S29–38.
Holmes GL, Stafstrom CE. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48(4):617–30.
Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–8.
Mete O, van der Kwast TH. Epithelioid angiomyolipoma: a morphologically distinct variant that mimics a variety of intra-abdominal neoplasms. Arch Pathol Lab Med. 2011;135(5):665–70.
Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Archive. 2008;452(2):119–32.
Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14(2):R251–8.
Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia. 2008;49(1):8–21.
Major P, Rakowski S, Simon MV, Cheng ML, Eskandar E, Baron J, et al. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009;50(1):147–54.
Weiner HL, Ferraris N, LaJoie J, Miles D, Devinsky O. Epilepsy surgery for children with tuberous sclerosis complex. J Child Neurol. 2004;19(9):687–9.
Kopp CM, Muzykewicz DA, Staley BA, Thiele EA, Pulsifer MB. Behavior problems in children with tuberous sclerosis complex and parental stress. Epilepsy Behavior. 2008;13(3):505–10.
Goh S, Butler W, Thiele EA. Subependymal giant cell tumors in tuberous sclerosis complex. Neurology. 2004;63(8):1457–61.
Adriaensen ME, Schaefer-Prokop CM, Stijnen T, Duyndam DA, Zonnenberg BA, Prokop M. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol. 2009;16(6):691–6.
Prather P, de Vries PJ. Behavioral and cognitive aspects of tuberous sclerosis complex. J Child Neurol. 2004;19(9):666–74.
Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol. 2004;19(9):680–6.
Tsao CY. Current trends in the treatment of infantile spasms. Neuropsychiatric Diseases and Treatment. 2009;5:289–99.
Ehninger D, de Vries PJ, Silva AJ. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res. 2009;53(10):838–51.
D'Agati E, Moavero R, Cerminara C, Curatolo P. Attention-deficit hyperactivity disorder (ADHD) and tuberous sclerosis complex. J Child Neurol. 2009;24(10):1282–7.
Winterkorn EB, Pulsifer MB, Thiele EA. Cognitive prognosis of patients with tuberous sclerosis complex. Neurology. 2007;68(1):62–4.
Johnson SR. Lymphangioleiomyomatosis. Eur Respir J. 2006;27(5):1056–65.
Yates JR. Tuberous sclerosis. Eur J Hum Genet. 2006;14(10):1065–73.
Franz DN. Non-neurologic manifestations of tuberous sclerosis complex. J Child Neurol. 2004;19(9):690–8.
Sooriakumaran P, Gibbs P, Coughlin G, Attard V, Elmslie F, Kingswood C, et al. Angiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treated. BJU Int. 2010;105(1):101–6.
McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133(2):507–16.
Jozwiak J, Jozwiak S, Oldak M. Molecular activity of sirolimus and its possible application in tuberous sclerosis treatment. Med Res Rev. 2006;26(2):160–80.
Isaacs H. Perinatal (fetal and neonatal) tuberous sclerosis: a review. Am J Perinatol. 2009;26(10):755–60.
Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC, et al. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18(1):56–62.
Napolioni V, Curatolo P. Genetics and molecular biology of tuberous sclerosis complex. Curr Genom. 2008;9(7):475–87.
Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804(3):433–9.
Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, et al. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22(18):2485–95.
de Vries PJ, Howe CJ. The tuberous sclerosis complex proteins: a GRIPP on cognition and neurodevelopment. Trends Mol Med. 2007;13(8):319–26.
Krishnan ML, Commowick O, Jeste SS, Weisenfeld N, Hans A, Gregas MC, et al. Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol. 2010;42(2):101–6.
Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27(21):5546–58.
Napolioni V, Moavero R, Curatolo P. Recent advances in neurobiology of tuberous sclerosis complex. Brain Dev. 2009;31(2):104–13.
Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784(1):116–32.
Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8(12):1727–34.
Crino PB. Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J Child Neurol. 2004;19(9):716–25.
Moavero R, Cerminara C, Curatolo P. Epilepsy secondary to tuberous sclerosis: lessons learned and current challenges. Childs Nerv Syst. 2010;26(11):1495–504.
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14(8):843–8.
Sampson JR. Therapeutic targeting of mTOR in tuberous sclerosis. Biochem Soc Trans. 2009;37(Pt 1):259–64.
Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51(1):27–36.
Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63(4):444–53.
Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2(67):e24.
Brown VI, Seif AE, Reid GS, Teachey DT, Grupp SA. Novel molecular and cellular therapeutic targets in acute lymphoblastic leukemia and lymphoproliferative disease. Immunol Res. 2008;42(1–3):84–105.
Akcakanat A, Singh G, Hung MC, Meric-Bernstam F. Rapamycin regulates the phosphorylation of rictor. Biochem Biophys Res Commun. 2007;362(2):330–3.
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–68.
Zeng Z, Sarbassov DD, Samudio IJ, Yee KW, Munsell MF, Ellen JC, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109(8):3509–12.
Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo). 1975;28(10):727–32.
Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59(1):3–16.
Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59(3):490–8.
Krueger DA, Care MM, Holland K, Agricola A, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–11.
Afinitor® (everolimus) tablets for oral administration [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp.; 2010.
Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–51.
Curatolo P, D'Argenzio L, Cerminara C, Bombardieri R. Management of epilepsy in tuberous sclerosis complex. Expert Rev Neurother. 2008;8(3):457–67.
Krueger DA, Franz DN. Current management of tuberous sclerosis complex. Paediatric Drugs. 2008;10(5):299–313.
Ess KC. Tuberous sclerosis complex: a brave new world? Curr Opin Neurol. 2010;23(2):189–93.
Jozwiak J, Sahin M, Jozwiak S, Kotulska K, Ploski R, Szperl M, et al. Cardiac rhabdomyoma in tuberous sclerosis: hyperactive Erk signaling. Int J Cardiol. 2009;132(1):145–7.
Kotulska K, Larysz-Brysz M, Grajkowska W, Jozwiak J, Wlodarski P, Sahin M, et al. Cardiac rhabdomyomas in tuberous sclerosis complex show apoptosis regulation and mTOR pathway abnormalities. Pediatr Dev Pathol. 2009;12(2):89–95.
Disclosure
Conflicts of interest: D.N. Franz: has received grant support for study drug and costs for conducting the research described in this paper, Novartis; has received a consulting fee or honorarium for conduct and design of clinical trials, Novartis; has received support for travel costs to meet with regulatory agencies, present research results, design protocols, Novartis; has received support for study medication, administrative support for study conduct, Novartis; has given expert testimony for various plaintiff's and defendant's attorneys; has received payment of travel and lodging expenses for invited lectures, not as part of a speaker's bureau; is on the speaker's bureau for UCB Pharma; B.D. Weiss: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franz, D.N., Weiss, B.D. Molecular Therapies for Tuberous Sclerosis and Neurofibromatosis. Curr Neurol Neurosci Rep 12, 294–301 (2012). https://doi.org/10.1007/s11910-012-0269-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-012-0269-4