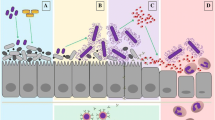
Abstract
Clostridium difficile infection (CDI) is a leading cause of nosocomial infections and the most important cause of health care-associated diarrhea worldwide. Standard treatment of CDI consists of modifying underlying antibiotic exposure, aggressive supportive measures, and therapy with specific antibiotics, most commonly metronidazole or vancomycin. This general approach to CDI has remained largely unchanged for decades. In an effort to improve outcomes and reduce recurrences of CDI, interest has been renewed in the development of nonantibiotic and adjunct approaches to therapy. In this review, we highlight some of these recent, resurrected, and novel nonantibiotic treatments.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bartlett JG: Historical perspectives on studies of Clostridium difficile and C. difficile infection. Clin Infect Dis 2008, 46(Suppl 1):S4-11.
Gerding DN: Clostridium difficile 30 years on: what has, or has not, changed and why? Int J Antimicrob Agents 2009, 33(Suppl 1):S2–8.
DuPont HL, Garey K, Caeiro JP, Jiang ZD: New advances in Clostridium difficile infection: changing epidemiology, diagnosis, treatment and control. Curr Opin Infect Dis 2008, 21:500–7.
Rupnik M, Wilcox MH, Gerding DN: Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 2009, 7:526–36.
Bobak DA: The molecular pathogenesis of Clostridium difficile -associated disease. Curr Infect Dis Rep 2008, 10:111–5.
Genth H, Dreger SC, Huelsenbeck J, Just I: Clostridium difficile toxins: more than mere inhibitors of Rho proteins. Int J Biochem Cell Biol 2008, 40:592–7.
Cohen SH, Gerding DN, Johnson S, et al: Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010, 31:431–455.
• Belyi Y, Aktories K: Bacterial toxin and effector glycosyltransferases. Biochim Biophys Acta 2010, 1800:134–43. This paper reviews the current knowledge regarding the structure and function of glycosyltransferases, including C. difficile toxins.
Pruitt RN, Chambers MG, Ng KK, et al: Structural organization of the functional domains of Clostridium difficile toxins A and B. Proc Natl Acad Sci USA 2010, 107:13467–72.
• Giesemann T, Egerer M, Jank T, Aktories K: Processing of Clostridium difficile toxins. J Med Microbiol. 2008, 57:690–6. This paper reviews current information regarding the intracellular processing and activation of C. difficile toxins.
Lyras D, O’Connor JR, Howarth PM, et al: Toxin B is essential for virulence of Clostridium difficile. Nature 2009, 458:1176–1179.
• Kuehne SA, Cartman ST, Heap JT, et al: The role of toxin A and toxin B in Clostridium difficile infection. Nature 2010, 467:711–3. The gene knockout study in the hamster model of CDI demonstrated that either one, or both, of C. difficile toxins could demonstrate cytotoxicity in vitro and virulence in vivo.
Bartlett JG: The case for vancomycin as the preferred drug for treatment of Clostridium difficile infection. Clin Infect Dis 2008, 46:1489–92.
Bartlett JG: New antimicrobial agents for patients with Clostridium difficile infections. Curr Infect Dis Rep 2009, 11:21–28.
Shah D, Dang MD, Hasbun R, et al: Clostridium difficile infection: update on emerging antibiotic treatment options and antibiotic resistance. Expert Rev Anti Infect Ther 2010, 8:555–64.
Thompson I: Clostridium difficile -associated disease: update and focus on non-antibiotic strategies. Age Ageing 2008, 37:14–8.
Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol 2009, 15:1554–80.
Koo HL, Garey KW, Dupont HL: Future novel therapeutic agents for Clostridium difficile infection. Expert Opin Investig Drugs 2010, 19:825–36.
Sekirov I, Russell SL, Antunes LC, Finlay BB, et al: Gut microbiota in health and disease. Physiol Rev 2010, 90:859–904.
Rea MC, Dobson A, O’Sullivan O, et al: Microbes and Health Sackler Colloquium: Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A 2010, [epub ahead of print] doi:10.1073/pnas.1001224107.
Chang JY, Antonopoulos DA, Kalra A, et al: Decreased diversity of the fecal microbiome in recurrent Clostridium difficile -associated diarrhea. J Infect Dis 2008, 197:435–8.
Pham M, Lemberg DA, Day AS: Probiotics: sorting the evidence from the myths. Med J Aust 2008, 188:304–8.
Quigley EM: Prebiotics and probiotics; modifying and mining the microbiota. Pharmacol Res 2010, 61:213–8.
Williams NT: Probiotics. Am J Health Syst Pharm 2010, 67:449–58.
Allen SJ, Okoko B, Martinez E, et al: Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev 2004, CD003048.
Guandalini S. Probiotics for children with diarrhea: an update. J Clin Gastroenterol 2008 Jul;42 Suppl 2:S53–7.
Chen CC, Kong MS, Lai MW, et al: Probiotics have clinical, microbiologic, and immunologic efficacy in acute infectious diarrhea. Pediatr Infect Dis J 2010, 29:135–8.
Hickson M, D’Souza AL, Muthu N, et al: Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007, 335:80.
Pillai A, Nelson R: Probiotics for treatment of Clostridium difficile -associated infection in adults. Cochrane Database Syst Rev 2008, CD004611.
McFarland LV: Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe 2009, 15:274–80.
Miller M: The fascination with probiotics for Clostridium difficile infection: lack of evidence for prophylactic or therapeutic efficacy. Anaerobe 2009, 15:281–4.
Hammerman C, Bin-Nun A, Kaplan M: Safety of probiotics: comparison of two popular strains. BMJ 2006, 333:1006–1008.
Munoz P, Bouza E, Cuenca-Estrella M, et al: Saccharomyces cerevisiae fungemia: an emerging infectious disease. Clin Infect Dis 2005, 40:1625–1634.
McFarland LV: Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 2010, 16:2202–2222.
Eiseman B, Silen W, Bascom GS, Kauvar AJ: Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44:854–9.
Aas J, Gessert CE, Bakken JS: Recurrent Clostridium difficile infection: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003, 36:580–585.
van Nood E, Speelman P, Kuijper EJ, Keller JJ: Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009, 14.
Rohlke F, Surawicz CM, Stollman N: Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010, 44:567–70.
Yoon SS, Brandt LJ: Treatment of refractory/recurrent C. difficile -associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010, 44:562–6.
Merrigan MM, Sambol SP, Johnson S, Gerding D, et al: New approach to the management of Clostridium difficile infection: colonisation with non-toxigenic C. difficile during daily ampicillin or ceftriaxone administration. Int J Antimicrob Agents 2009, 33(Suppl 1):S46–50.
Meader E, Mayer MJ, Gasson MJ, et al: Bacteriophage treatment significantly reduces viable Clostridium difficile and prevents toxin production in an in vitro model system. Anaerobe 2010, [Epub ahead of print] PubMed PMID: 20816997.
Kyne L, Warny M, Qamar A, Kelly CP: Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001, 357:189–193.
Leav BA, Blair B, Leney M, et al: Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine 2010, 28:965–969.
O’Horo J, Safdar N: The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int J Infect Dis 2009, 13:663–7.
Abougergi MS, Broor A, Lui W, Jaar BG: Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature. J Hosp Med 2010, 5:E1–9.
• Abougergi MS, Kwon JH: Intravenous immunoglobulin for the treatment of Clostridium difficile infection: A Review. Dig Dis Sci. 2010, [Epub ahead of print] PubMed PMID: 20924675. This paper reviews the results of the currently published case reports and clinical trials using IVIg as adjunct therapy of CDI.
Salcedo J, Keates S, Pothoulakis C, et al: Intravenous immunoglobulin therapy for severe Clostridium difficile infection. Gut 1997, 41:366–370.
Beales IL: Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002, 51:456.
Wilcox MH: Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother 2004, 53:882–884.
Pherson S, Rees CJ, et al: Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum 2006, 49:640–5.
Juang P, Skledar SJ, Zgheib NK, et al. Clinical outcomes of intravenous immune globulin in severe Clostridium difficile -associated diarrhea. Am J Infect Control 2007, 35:131–137.
•• Lowy I, Molrine DC, Leav BA, et al: Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010, 362:197–205. This randomized, double-blind, placebo-controlled study showed that infusion of CDA1/CDB1 significantly decreased the recurrence rate of CDI in the study population.
Babcock GJ, Broering TJ, Hernandez HJ, et al: Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile -induced mortality in hamsters. Infect Immun 2006, 74:6339–6347.
Lee BY, Popovich MJ, Tian Y, et al. The potential value of Clostridium difficile vaccine: an economic computer simulation model. Vaccine 2010, 28:5245–53.
Kaslow DC. Emerging concepts for developing vaccines for Clostridium difficile and Methicillin-Resistant Staphylococcus aureus. Annu Rev Med 2011 Jan 27. [Epub ahead of print] PubMed PMID: 20707676.
Taylor NS, Bartlett JG: Binding of Clostridium difficile cytotoxin and vancomycin by anion-exchange resins. J Infect Dis 1980, 141:92–7.
Weiss K: Toxin-binding treatment for Clostridium difficile : a review including reports of studies with tolevamer. Int J Antimicrob Agents 2009, 33:4–7.
Sorg JA, Sonenshein AL: Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid . J Bacteriol. 2010, 192:4983–90.
Giel JL, Sorg JA, Sonenshein AL, Zhu J: Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One 2010, :e8740.
Louie TJ, Peppe J, Watt CK, et al: Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile- associated diarrhea . Clin Infect Dis 2006, 43:411–420.
Hinkson PL, Dinardo C, DiCiero D, et al: Tolevamer, an anionic polymer, neutralizes toxins produced by the BI/027 strains of Clostridium difficile . Antimicrob Agents Chemother 2008, 52:2190–2195.
Baines SD, Freeman J, Wilcox MH: Tolevamer is not efficacious in the neutralization of cytotoxin in a human gut model of Clostridium difficile infection. Antimicrob Agents Chemother 2009, 53:2202–2204.
Peppe J, Porzio A, Davidson DM: A new formulation of tolevamer, a novel nonantibiotic polymer, is safe and well-tolerated in healthy volunteers: a randomized phase I trial. Br J Clin Pharmacol 2008, 66:102–9.
Noblett SE, Welfare M, Seymour K: The role of surgery in Clostridium difficile colitis. BMJ 2009, 338:b1563.
Butala P, Divino CM: Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg 2010, 200:131–5.
Gash K, Brown E, Pullyblank A: Emergency subtotal colectomy for fulminant Clostridium difficile colitis-is a surgical solution considered for all patients? Ann R Coll Surg Engl 2010, 92:56–60
Barczak AK, Hung DT. Productive steps toward an antimicrobial targeting virulence. Curr Opin Microbiol . 2009, 12:490–6.
Alvarez Z, Abel-Santos E. Potential use of inhibitors of bacteria spore germination in the prophylactic treatment of anthrax and Clostridium difficile -associated disease. Expert Rev Anti Infect Ther 2007, 5:783–92.
• Burns DA, Heap JT, Minton NP: Clostridium difficile spore germination: an update. Res Microbiol 2010, [Epub ahead of print] PubMed PMID: 20863888. This paper reviews the current concepts of the process of spore germination by C. difficile.
Ochsner UA, Bell SJ, O’Leary AL, et al: Inhibitory effect of REP3123 on toxin and spore formation in Clostridium difficile , and in vivo efficacy in a hamster gastrointestinal infection model. J Antimicrob Chemother 2009, 63:964–71.
Carneiro BA, Fujii J, Brito GA, et al: Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro . Infect Immun 2006, 74:81–7.
Maciel AA, Oriá RB, Braga-Neto MB, et al: Role of retinol in protecting epithelial cell damage induced by Clostridium difficile toxin A. Toxicon 2007, 50:1027–40.
• Ng J, Hirota SA, Gross O, et al: Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 2010, 139:542–52. This study showed that several of the proinflammatory and cytotoxic effects of TcdA and TcdB are mediated via the inflammasome-mediated upregulation of interleukin-1.
Franchi L, Eigenbrod T, Nunoz-Planillo R, Nunez G: The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009, 10:241–7.
• Abdeen SJ, Swett RJ, Feig AL: Peptide Inhibitors Targeting Clostridium difficile Toxins A and B. ACS Chem Biol 2010, [Epub ahead of print] PubMed PMID: 20863124. This paper used phage display libraries to identify polypeptide inhibitors of the catalytic domain of TcdA in vitro.
Jank T, Ziegler MO, Schulz GE, Aktories K: Inhibition of the glucosyltransferase activity of clostridial Rho/Ras-glucosylating toxins by castanospermine. FEBS Lett 2008, 582:2277–82.
Hirota SA, Fines K, Traboulsi D: Hypoxia-inducible factor signaling provides protection in Clostridium difficile- induced intestinal injury. Gastroenterology. 2010, 139:259–69.
• Giesemann T, Guttenberg G, Aktories K: Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology 2008, 134:2049–58. This study showed that certain human defensins bound with, and partially inactivated, TcdB, but not TcdA.
Disclosure
Conflicts of interest: N. Suwantarat—none; D. Bobak—none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suwantarat, N., Bobak, D.A. Current Status of Nonantibiotic and Adjunct Therapies for Clostridium difficile Infection. Curr Infect Dis Rep 13, 21–27 (2011). https://doi.org/10.1007/s11908-010-0155-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-010-0155-7