Abstract
Purpose of Review
Our understanding of beta blockers in liver cirrhosis has transformed over the last 40 years. However, questions remain over their safety in acute decompensation and acute on chronic liver failure. Since these conditions are associated with significant morbidity and mortality, a critical appraisal of recent literature is imperative to help guide clinicians.
Recent Findings
The latest BAVENO guidelines now recommend carvedilol in all patients with clinically significant portal hypertension to prevent decompensation. There is significant data which shows safety of beta blocker use in decompensated cirrhosis but concerns remain in refractory ascites. There is also a short-term mortality benefit demonstrated in acute on chronic liver failure.
Summary
With the latest guidelines and recent evidence, it seems beta blocker use will continue to increase. Future studies should aim to identify biomarkers that can determine who will benefit from beta blockers and help guide therapy.
Similar content being viewed by others
Introduction
It was just over 40 years ago that the landmark study by Lebrec et al. demonstrated for the first time that propranolol significantly reduced portal hypertension (PH) in liver cirrhosis and therefore could prevent recurrent variceal bleeding [1]. Since then, there has been a wealth of published literature showing the efficacy of non-selective beta-blockers (NSBBs) in primary and secondary prevention of variceal bleeding, reducing the occurrence of spontaneous bacterial peritonitis (SBP), providing survival benefit and potentially reducing hepatocellular carcinoma (HCC) [2,3,4,5,6].
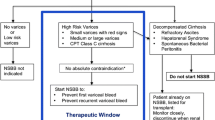
However, Sersté et al.raised concerns that NSBBs could increase mortality in individuals with cirrhosis and refractory ascites [7]. Subsequent research led to the development of the “therapeutic window hypothesis” which suggested that NSBBs should not be used in early cirrhosis without significant varices where sympathetic nervous system activity (SNS) is near normal. In addition, they should not be used in end-stage cirrhosis where they could increase mortality by reducing cardiac contractility and mean arterial pressure (MAP) [8]. However, in recent years, several studies have suggested that NSBBs can be used safely in these settings and possibly provide survival benefit in most scenarios in decompensated cirrhosis [9, 10].
This paints the picture for the current controversy of NSBBs in cirrhosis. Questions exist over whether NSBBs should be used in advanced cirrhosis and in all individuals with clinically significant portal hypertension (CSPH), which is defined as a portal pressure ≥ 10 mmHg [11]. Furthermore, uncertainty remains over whether it is safe to continue NSBBs in acute decompensation (AD) and acute-on-chronic liver failure (ACLF), and if so, what criteria should be used to stop and when to re-start therapy? Once on treatment, the optimal method to monitor NSBB response has not been determined. Whilst there is increasing acceptance that invasive monitoring in the form of hepatic venous pressure gradient (HVPG) cannot be used routinely, questions remain over the accuracy of non-invasive monitoring. This review will aim to address some of these issues by focussing on recent research in the last 3 years and will highlight important questions that still remain unanswered.
Role of NSBBs in Preventing Decompensation
One of the most significant recent publications is the PREDESCI trial, which was a multi-centre, double-blind, randomised controlled trial (RCT) that aimed to determine whether NSBBs provided benefit in terms of preventing decompensation and mortality in individuals with compensated cirrhosis and CSPH [12••]. The study involved 201 patients with a follow-up over 37 months. It is the first study to demonstrate that treatment with NSBBs was associated with a significant reduction in decompensation or death compared to placebo (16% vs 27%, p = 0.041). This was predominantly through a reduction in the development of ascites (9% vs 20%), which is the most prevalent decompensating event and for which, until now, there has been no preventative pharmacotherapy. Carvedilol was shown to have greater portal pressure-reducing effects compared to traditional NSBBs (16% vs 10%, p = 0.04), despite being in the group that did not respond to IV propranolol at time of HVPG measurement. Indeed, the recent BAVENO VII guidelines now advocate that NSBBs should be considered for all patients with CSPH to prevent decompensation [13••]. With such a significant recommendation to change practice, one could query whether these changes go too far. Questions have been raised over the applicability of the PREDESCI findings to clinical practice. The study involved invasive HVPG monitoring to diagnose CSPH. In addition, the aetiology for most patients in this study was hepatitis C and questions remain over whether these findings hold true for other populations that have predominantly alcohol- and non-alcohol steatohepatitis (NASH)–related cirrhosis.
McDowell et al.performed a retrospective analysis of 152 patients that had been recruited to an RCT of carvedilol versus variceal band ligation (VBL) in preventing first variceal bleed [14•]. Whilst median survival was significantly higher in the carvedilol cohort (7.8 years vs 4.2 years, p = 0.03), no difference was noted between transplant free-survival, liver-related mortality and decompensation events suggesting that the survival benefit observed in the patients treated with carvedilol was not purely liver related. These intriguing findings warrant prospective studies.
Previous studies have suggested that there may be a role for NSBBs in preventing HCC. Of note, Wijarnpreecha et al.performed a retrospective cohort study comprising 107,428 patients with cirrhosis. The 100-month cumulative incidence of HCC was lower in all NSBB groups: carvedilol (11.2%) vs no NSBB (15.7%) and propranolol (26.2%) vs no NSBB (28.8%) [15•]. A subgroup analysis of patients with decompensated cirrhosis showed that the protective effect of NSBBs remained. The underlying mechanism is not known but hypothesised to be through inhibition of angiogenesis and carcinogenesis pathways. Future prospective studies should assess whether this benefit is observed across all severities of liver disease.
Given the current evidence, whilst some clinicians are reluctant, there is a strong argument for the use of NSBBs in all compensated patients with CSPH. However, future studies are needed to evaluate the role of NSBBs in a population more weighted with alcohol and NASH aetiologies. In addition, they should make use of non-invasive measures as opposed to HVPG to guide therapy and determine potential benefits.
Role of NSBBs in Decompensated Cirrhosis
As previously highlighted, the upper limit of the therapeutic window of NSBB in treating patients with decompensated cirrhosis remains controversial. Tergast et al.attempted to define this with a retrospective study of 624 patients with decompensated cirrhosis and ascites [16••]. They showed that patients with a MAP ≥ 65 mmHg had a superior 28-day transplant-free survival on NSBB (p = 0.021), but this benefit was lost in patients with a MAP < 65 mmHg. Indeed, early discontinuation of NSBB was associated with lower survival in patients with a MAP ≥ 65 mmHg (p = 0.004). A subset of the population with SBP was also analysed, and in those with a MAP ≥ 65 mmHg a survival benefit was observed with NSBBs (p = 0.008). A non-significant trend was also noted towards increased incidence of acute kidney injury (AKI) in those with SBP on NSBBs (HR: 2.957, p = 0.085), but this was not the case in those with a MAP ≥ 65 mmHg. A subsequent analysis showed that using a cut-off for systolic blood pressure of > 90 mmHg was comparable to a MAP threshold of 65 mmHg, which is consistent with current guidelines [17]. A limitation of this study is that 58% of the patients on NSBBs were on propranolol with a median dose of 30 mg/day. Other studies which have shown increased mortality with NSBBs have used higher doses of propranolol which may have skewed the positive findings seen here.
Ngwa et al. performed a retrospective analysis of 170 patients and observed a trend towards lower 90-day mortality in the NSBB cohort versus the non-NSBB group (6% vs 15% p = 0.06), despite having statistically higher Child Pugh and MELD scores [18]. An increased risk of AKI was noted in the NSBB group (22% vs 11%, p = 0.048). In the study, 44 patients had refractory ascites or a history of SBP and there was no difference in 90-day mortality and AKI between this cohort and the rest of the population. The study showed no survival benefit of NSBB beyond 90 days. Whilst a small study, this seems to demonstrate safety data of NSBBs in patients with refractory ascites. In contrast, the study by Tellez et al.provided different results. They performed a prospective study of 18 patients with diuretic responsive ascites (DRA) and 20 with refractory ascites (RA) [19••]. Patients were treated with propranolol for 4 weeks with baseline and repeat measurements performed of Doppler echocardiography, as well as haemodynamic assessments of hepatic, kidney and cardiopulmonary systems. The study assessed overall systolic function using the ejection intraventricular pressure difference (EIVPD) as a load-independent marker. Propranolol caused a significant reduction in EIPVD in the RA compared to DRA cohorts (− 20% vs − 2%, p < 0.01). Renal perfusion pressure (RPP) reduced in both groups but dropped below 65 mmHg, the threshold for autoregulation in 55% in the RA group vs 12% in the DRA group (p = 0.01). This was reflected by a significant decrease in eGFR and increase in creatinine in the RA group (0.24 mg/dl, p = 0.01). This study suggests that in refractory ascites, NSBBs may blunt cardiac output and impair renal perfusion and therefore should be avoided. Whilst the study and haemodynamic measurements involved were robust, the numbers were small.
A recent meta-analysis also aimed to provide further clarity about the safety of NSBBs in decompensated cirrhosis and ascites [20]. In total, 8 studies with a total of 3267 patients were analysed. A sub-cohort analysis of patients with severe or refractory ascites showed similar mortality in the NSBB group (33.3%) compared with the non-NSBB group (32.1%). Three studies were deemed to be good quality and 5 fair quality. However, there was significant study heterogeneity (I2 = 87%) which led the authors giving a GRADE rating of the evidence as “very low”. Hence, data from this meta-analysis are not possible to interpret.
Overall, whilst some studies have demonstrated safety of NSBBs in decompensated cirrhosis including in SBP, the literature would suggest ongoing concern for its use in patients with refractory ascites, particularly with regard to renal impairment. A MAP of 65 mmHg/systolic of 90 mmHg should be used to reduce or withhold NSBBs. Prospective studies should address whether NSBBs prevent further decompensation events in already decompensated patients.
Role of NSBBs in ACLF
Clinicians are understandably cautious to use NSBBs in ACLF given that it is a hyperinflammatory condition associated with circulatory dysfunction and multiorgan failure. Mookerjee et al. previously showed that in patients receiving NSBBs at the time of hospitalisation with ACLF, 28-day survival was significantly higher than those that were not receiving NSBBs [21]. More recently, the study by Tergast et al.which has been previously discussed also studied 254 (41%) individuals with ACLF.[16••] Patients receiving NSBBs prior to hospitalisation had an increased 28-day transplant-free survival in a multivariate analysis (p = 0.004). As per the decompensated cirrhosis cohort, a MAP ≥ 65 mmHg conferred survival benefit with no difference observed with a MAP < 65 mmHg. Early discontinuation of NSBB in the whole cohort led to reduced survival (p = 0.004) and there was no association with renal impairment. This study also showed some positive impact of NSBBs with regard to lung and coagulation failure, suggesting benefits beyond purely reducing portal pressure. There is increasing evidence of the anti-inflammatory properties of NSBBs, reflected in this study. The NSBB cohort had lower white blood cells (WBC) (p = 0.014). However, no difference in CRP was noted. Further limitations of this study are its retrospective nature and that ACLF was only assessed at two time points. Therefore, some patients may have been missed out of the analysis.
The Sarin group was the first to do prospective randomised control trial of NSBBs in ACLF [22••]. A total of 136 patients with ACLF and HPVG ≥ 12 mmHg were randomised to either carvedilol or placebo. When the carvedilol group was compared to the control group censored at 28 days, there was a reduction in (i) mortality (10.6% vs 24.3%, p = 0.044), (ii) AKI development (13.6% vs 35.7%, p = 0.012), (iii) development of SBP (6.1% vs 21.4%, p = 0.013) and (iv) variceal progression (11.1% vs 32.6%, p = 0.021). In the 2 weeks after commencing treatment, increases in ACLF grade were noted in 22.9% of controls and 6.1% of those on carvedilol (p = 0.007). However, this benefit was lost at 90 days [21]. Although this data is interesting, it is difficult to understand how this effect was achieved by carvedilol and before this data is used in clinical practice, further adequately powered, multicentre trial data are needed.
Preference of Type of NSBB and Dose Titration
The mechanisms of how NSBBs work in cirrhosis patients is demonstrated in Fig. 1 [23]. Sharma et al. conducted a post hoc analysis of a RCT comparing carvedilol with propranolol following an acute variceal bleed [24]. They demonstrated reduced incidence of ascites in the carvedilol group (40% vs 69.5%, p = 0.04). However, there were only 25 patients in the carvedilol arm and 23 in the propranolol arm. Therefore, the results should be interpreted with caution.
A recent meta-analysis by Sharma et al. compared carvedilol, traditional NSBBs and VBL as individual and combination therapies [25]. Whilst traditional NSBBs were associated with a higher odds ratio for bleeding than carvedilol (OR 3.05 [CI 1.20–7.74]), no difference was observed in mortality. However, the authors do note there was a lack of RCTs including carvedilol and suggested a need for more prospective studies.
There is one recent prospective RCT carried out by Kalambokis et al.involving 96 patients on propranolol for variceal bleeding prophylaxis [26••]. Patients were randomised in a 2:1 ratio to switch to carvedilol at 12.5 mg/day (n = 64) or continue propranolol (n = 32). At 12 months, the carvedilol group showed a significant improvement in systemic vascular resistance, glomerular filtration rate and renal blood flow with significant decreases in plasma renin activity and plasma noradrenaline. After 2 years, the incidence of further decompensation was lower in carvedilol group compared to the propranolol group (10.5% vs 35.9%, p = 0.003). This was associated with improved survival (86% vs 64.1%, p = 0.01). However, the numbers are small with ascites being the most common decompensation event in follow-up and this only being detected in 5 patients in the propranolol group and 2 in the carvedilol group.
Therapeutic heart rate reduction and blood pressure still seem to be the mainstay of titrating NSBBs, as well as the main outcome measures used in clinical trials to determine adequate dosing. Previous studies have demonstrated that lowering HR to 55–65 beats per minute (BPM) can improve myocardial oxygen demand and coronary perfusion with independent effects on portal hypertension [27]. Indeed, a previous meta-analysis showed a therapeutic of 5 bpm with NSBBs could provide a 14% reduction in mortality [28]. More recent data suggests the importance of monitoring blood pressure in guiding therapy rather than heart rate. Table 1 shows some potential options to help guide NSBB therapy in the future.
In summary, based on the current evidence, the first choice NSBB should therefore be carvedilol titrated to a maximum of 12.5 mg/day. Higher doses may lack additional portal pressure reduction, whilst compromising tolerability due to potential systemic hypotension [12••]. Indeed, the BAVENO VII guidelines advise carvedilol as the preferred choice of NSBB in compensated cirrhosis due to (i) superior reduction in HPVG, (ii) greater benefit in preventing decompensation and (iii) greater tolerability [13••].
Stopping and Re-starting NSBBs
There is limited literature addressing outcomes of stopping and re-starting NSBBs. Bhutta et al.performed a retrospective study of 716 patients of which 43% were on NSBB and 51% had refractory ascites [9]. They showed that NSBB use in hospitalised patients with ascites was not associated with increased mortality. Forty-nine percent of patients discontinued NSBBs during admission because of low blood pressure in the context of infection or AKI with no increase in mortality. This cohort had a mean systolic blood pressure of 103 mmHg at discontinuation of NSBB, compared to 114 mmHg on admission, which is higher than the EASL recommendation of 90 mmHg for reducing or stopping NSBBs [17]. However, there was a higher incidence of infection and renal impairment in those that discontinued NSBBs, and it is likely that there was a combination of factors which led to NSBB discontinuation. Forty percent of patients were re-commenced on NSBB prior to discharge which was predominantly driven by an increase in mean MAP to 84mmg (± 11) at re-initiation. However, mean follow-up for the study was only 14 ± 24 days and therefore long-term effects of this strategy are not known.
There is increasing evidence that cardiac indices could be used to guide NSBB therapy. Alvarado-Tapias et al.performed a retrospective analysis and demonstrated that in a cohort of patients with cirrhosis (n = 190), those who died had significantly lower cardiac output (CO) on NSBB and that CO could independently predict death (C-index 0.74) [34•]. The authors determined a cut off CO of 5L/min, below which a significantly higher mortality was observed (HR 0.44, p = 0.004), with a sensitivity of 81% and specificity of 60%. Indeed, this was retrospective study with no control arm and only 1–3-month follow-up. A further study by Giannelli et al.retrospectively analysed 584 patients, of whom 50% were receiving NSBBs and 33% had refractory ascites [35•]. Right heart catheterisation was performed to determine left ventricular stroke work index (LVSWI) which considers not only cardiac output but also arterial pressure providing a global assessment of cardiac function. In a multivariate analysis, they observed that receiving NSBBs with LSWI cut-off < 64.1 g-m/m led to higher risk of waiting list mortality than those not on NSBBs (p = 0.0008). Whilst these data provide guidance regarding withdrawal of NSBBs, this is a single-centre study and LVSWI has not been studied extensively in cirrhosis and needs further validation. In addition, both studies discussed here required invasive measurements via cardiac catheterisation. Future studies should prospectively analyse cardiac indices using non-invasive techniques such as 2D echocardiography or MRI.
Future Research
The role Systemic Inflammation as a Target
It is known that ACLF and decompensated cirrhosis are associated with significant systemic inflammation and that this is associated with the severity of liver disease, portal hypertension–associated complications and mortality [37, 38]. Several studies have demonstrated that NSBBs can reduce systemic inflammation [21, 39]. It has been speculated that their mechanism may involve reducing gut permeability by reducing splanchnic blood flow and hence intestinal wall oedema, as well as increasing intestinal transit. Jachs et al. recently performed a retrospective study of 307 patients including 231 patients with decompensated disease [33••]. They showed a significant reduction in WBC with NSBB therapy (median: − 2 × 109, p = 0.011), independent of HPVG response and this was most pronounced in Child–Pugh C patients. These changes significantly correlated with a reduction in CRP, procalcitonin and IL-6. The group also showed that a reduction of WBC ≥ 15% was associated with a reduced risk of further decompensation (HR 0.694, p = 0.038) and liver-related mortality (HR 0.561, p = 0.013). However, prospective, long-term studies with control arms are required to develop our understanding and potential therapeutic targets of systemic inflammation.
HCC and Portal Vein Thrombosis (PVT)
As stated previously in the review, retrospective studies have showed that NSBBs may reduce the incidence of HCC. Whilst not the focus of this review, some historic studies have suggested that NSBBs can increase incidence of PVT, although the prospective PREDESCI study did not show this [12••]. More data are needed to substantiate these findings.
Non-invasive Biomarkers/Imaging
A robust biomarker for non-haemodynamic response and guiding NSBB therapy remains elusive. Recently Jachs et al.performed a retrospective analysis of 159 patients with clinically stable decompensated cirrhosis [40••]. The patients had paired HVPG/von Willebrand factor (VWF) measurements prior to and then subsequently whilst they were receiving NSBBs. Those who had a ≥ 5% decrease in VWF were deemed as “VWF-responders” versus those who did not, “VWF-non-responders”. The VWF responders had significantly reduced CRP and procalcitonin levels compared with the non-responders suggesting reduced inflammation. In a multivariate analysis, the VWF-responder group was associated with a reduced risk of; decompensation (adjusted hazard ratio [aHR] 0.55, p = 0.20), ACLF (aHR 0.40, p = 0.007), AKI (aHR 0.37, p = 0.01) and liver-related death (aHR 0.332, p < 0.001). This suggests vWF may be a useful biomarker to guide who will benefit from NSBB therapy. The limitations of this study are its retrospective nature. In addition, the vWF responders had a significantly higher proportion of patients on carvedilol and it is unclear how much of the benefit seen was due to NSBB class.
There is a wealth of data addressing the utility of imaging in assessing fibrosis progression and portal hypertension [41, 36]. Some recent evidence suggests that whilst liver stiffness (LS) is well validated, it may not be as effective as splenic stiffness (SS) in determining NSBB response. Marasco et al. performed a prospective study to assess haemodynamic response in patients on NSBBs [42]. They showed that changes in HVPG did not correlate with LS (r = 0.12, p = 0.65) but did correlate with SS (r = 0.78, p < 0.0001). A reduction in SS ≥ 10% correlated with being a HVPG responder (HVPG reduction ≤ 10% or < 12 mmHg). However, the study was small with only 20 patients included with no multivariate analysis. These findings were supported by a larger study done by Kim et al. assessing 106 patients on carvedilol using acoustic radiation forced impulse (ARFI) [30]. Baseline and repeat measurements of HVPG, LS and SS on carvedilol demonstrated in a multivariate analysis that only a change in SS was a predictor of haemodynamic response to NSBBs (≈80%).
Potential biomarkers for future studies include copeptin and asymmetric dimethylarginine (ADMA). Copeptin has demonstrated a predictive role in ascites formation, HE and survival, and ADMA has shown correlation with portal hypertension and mortality [31, 32]. Future studies should be prospective in nature and aim to validate biomarkers/imaging and help guide the following treatment decisions: who to treat with NSBBs, titrating dosages and defining when to stop/re-start treatment.
Future of NSBB Use
Currently, screening endoscopy is recommended in individuals at risk of high-risk varices defined by liver stiffness (LS) ≥ 20 kPa or platelet count ≤ 150 × 109/L [13••]. A new paradigm proposed by Garcia-Tsao et al. involves non-invasive measures to identify those with CSPH (either LS > 25 kPa or LS > 20–25 kPa and platelets < 150 × 109/L, or presence of varices or portal-systemic collaterals on imaging). Those who fulfil criteria would be treated with carvedilol with the aim of preventing all-cause decompensation. Those who did not fulfil the criteria would have annual LS and platelet measurements [29]. Many countries were forced to do this during the pandemic due to resource limitation. Not only would this be cost-effective in terms of reducing screening endoscopies but it would also be preferable to patients. The exceptions to this would be individuals who are intolerant of NSBBs or present with decompensated cirrhosis for which the initial paradigm stands. Whilst a cut-off LS > 25 kPa is valid for alcohol related liver disease, hepatitis B and C, it performed poorly in obese patients with non-alcoholic steatohepatitis (detecting only 62.8% of CSPH) and in patients with cholestatic disorders such as primary biliary cholangitis [43].
The jury on the universal use of carvedilol 12.5 mg OD in all individuals with CSPH to prevent decompensation is out and is a matter of ongoing debate despite the Baveno VII guidance. Whilst there is significant safety data in decompensated cirrhosis and we would advocate carvedilol use, concerns remain for patients with refractory ascites and risk of AKI. It is clear that NSBBs should be held and/or reduced when the MAP is < 65 mmHg or systolic blood pressure is < 90 mmHg and re-introduced cautiously as the blood pressure improves.
Conclusion
This review highlights that we are in a new era of NSBB management. Recent evidence has shown safety in clinical settings where historic literature would suggest stopping therapy. However, we must not become overzealous, and concern does remain in certain scenarios that have been discussed in this paper. The golden ticket for researchers in this field remains a non-invasive biomarker to guide NSBB therapy. It is an exciting time, and we look forward to future studies that will hopefully clarify the unanswered questions that remain.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lebrec D, Corbic M, Nouel O, Benhamou J-P. Propranolol—a medical treatment for portal hypertension? Lancet. 1980;316(8187):180–2.
Wijarnpreecha K, Li F, Xiang Y, Xu X, Zhu C, Maroufy V, et al. Nonselective beta-blockers are associated with a lower risk of hepatocellular carcinoma among cirrhotic patients in the United States. Aliment Pharmacol Ther. 2021;54(4):481–92.
Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, et al. β-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int. 2009;29(8):1189–93.
Lo G-H, Chen W-C, Lin C-K, Tsai W-L, Chan H-H, Chen T-A, et al. Improved survival in patients receiving medical therapy as compared with banding ligation for the prevention of esophageal variceal rebleeding. Hepatology. 2008;48(2):580–7.
Jakab SS, Garcia-Tsao G. Evaluation and management of esophageal and gastric varices in patients with cirrhosis. Clin Liver Dis. 2020;24(3):335–50.
Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. The Lancet. 2014;383(9930):1749–61.
Sersté T, Melot C, Francoz C, Durand F, Rautou P-E, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52(3):1017–22.
Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of β-blockers improve survival of patients with cirrhosis during a window in the disease: Figure 1. Gut. 2012;61(7):967–9.
Bhutta AQ, Garcia-Tsao G, Reddy KR, Tandon P, Wong F, O’Leary JG, et al. Beta-blockers in hospitalised patients with cirrhosis and ascites: mortality and factors determining discontinuation and reinitiation. Aliment Pharmacol Ther. 2018;47(1):78–85.
Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: Post Hoc analysis of three randomized controlled trials with 1198 patients. Hepatology. 2016;63(6):1968–76.
Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481–8.
Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393(10181):1597–608. First multi-centre, double blinded RCT that demonstrates NSBBs can reduce risk of death and decompensation, predominantly through a reduction in ascites.
de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959–974. https://doi.org/10.1016/j.jhep.2021.12.022. Epub 2021 Dec 30. BAVENO VII update on portal hypertension guidelines.
McDowell HR CCTDSAFEHP. Carvedilol is associated with improved survival in patients with cirrhosis: a long-term follow-up study. Aliment Pharmacol Ther. 2021;54(4):531–9. Retrospective study showing survival benefit of carvedilol compared to variceal band ligation, but no benefit in decompensation rates and liver related mortality suggesting non-liver related benefits.
Wijarnpreecha K, Li F, Xiang Y, Xu X, Zhu C, Maroufy V, et al. Nonselective beta‐blockers are associated with a lower risk of hepatocellular carcinoma among cirrhotic patients in the United States. Aliment Pharmacol Ther. 2021;54(4):481–92. Retrospective study of over 100,000 patients suggesting a protective effect of NSBBs in reducing HCC incidence, with this effect being maintained in the decompensated cirrhosis cohort.
Tergast TL, Kimmann M, Laser H, Gerbel S, Manns MP, Cornberg M, et al. Systemic arterial blood pressure determines the therapeutic window of non-selective beta blockers in decompensated cirrhosis. Aliment Pharmacol Ther. 2019;50(6):696–706. Retrospective study demonstrating that NSBBs improve 28-day transplant free survival in decompensated cirrhosis with ascites and ACLF. This benefit is lost when MAP falls below 65mmHg.
Angeli P, Bernardi M, Villanueva C, Francoz C, Mookerjee RP, Trebicka J, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–60.
Ngwa T, Orman E, Gomez EV, Vuppalanchi R, Kubal C, Chalasani N, et al. Non-selective beta blocker use is associated with improved short-term survival in patients with cirrhosis referred for liver transplantation. BMC Gastroenterol. 2020;20(1):4.
Téllez L, Ibáñez-Samaniego L, Pérez del Villar C, Yotti R, Martínez J, Carrión L, et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol. 2020;73(6):1404–14. Prospective study with cardiac, hepatic and renal haemodynamics showing that NSBBs blunt cardiac output and causes renal impairment in refractory ascites.
Wong RJ, Robinson A, Ginzberg D, Gomes C, Liu B, Bhuket T. Assessing the safety of beta-blocker therapy in cirrhosis patients with ascites: A meta-analysis. Liver Int. 2019;39(6):1080–8.
Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, Macnaughtan J, Bendtsen F, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64(3):574–82.
Kumar M, Kainth S, Choudhury A, Maiwall R, Mitra LG, Saluja V, et al. Treatment with carvedilol improves survival of patients with acute-on-chronic liver failure: a randomized controlled trial. Hepatol Int. 2019;13(6):800–13. Prospective RCT in ACLF demonstrating that carvedilol, when censored at 28 days, reduces mortality, AKI development, SPB incidence and variceal progression.
Kim SG, Kim TY, Sohn JH, Um SH, Seo YS, Baik SK, et al. A Randomized, Multi-center, open-label study to evaluate the efficacy of carvedilol vs. propranolol to reduce portal pressure in patients with liver cirrhosis. Am J Gastroenterol. 2016;111(11):1582–90.
Sharma S, Agarwal S, Gunjan D, Kaushal K, Anand A, Mohta S, et al. Long-term outcomes with carvedilol versus propranolol in patients with index variceal bleed: 6-year follow-up study. J Clin Exp Hepatol. 2021;11(3):343–53.
Sharma M, Singh S, Desai V, Shah VH, Kamath PS, Murad MH, et al. Comparison of therapies for primary prevention of esophageal variceal bleeding: a systematic review and network meta-analysis. Hepatology. 2019;69(4):1657–75.
Kalambokis GN, Christaki M, Tsiakas I, Despotis G, Fillipas-Ntekouan S, Fotopoulos A, et al. Conversion of propranolol to carvedilol improves renal perfusion and outcome in patients with cirrhosis and ascites. J Clin Gastroenterol. 2021;55(8):721–9. Prospective RCT demonstrating that carvedilol improves renal perfusion, improves survival and reduces decompensation events in comparison to propranolol.
Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology. 2010;51(6):2214–8.
McAlister FA. Meta-analysis: β-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150(11):784.
Garcia-Tsao G, Abraldes JG. Nonselective beta-blockers in compensated cirrhosis: preventing variceal hemorrhage or preventing decompensation? Gastroenterology. 2021;161(3):770–3.
Kim HY, So YH, Kim W, Ahn D-W, Jung YJ, Woo H, et al. Non-invasive response prediction in prophylactic carvedilol therapy for cirrhotic patients with esophageal varices. J Hepatol. 2019;70(3):412–22.
Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45(1):62–71.
Shigefuku R, Iwasa M, Eguchi A, Tamai Y, Yoshikawa K, Sugimoto R, et al. Serum copeptin level is a biomarker associated with ascites retention and the formation of a portosystemic shunt in chronic liver disease. J Gastroenterol Hepatol. 2021;36(4):1006–14.
Jachs M, Hartl L, Schaufler D, Desbalmes C, Simbrunner B, Eigenbauer E, et al. Amelioration of systemic inflammation in advanced chronic liver disease upon beta-blocker therapy translates into improved clinical outcomes. Gut. 2021;70(9):1758–67. Retrospective study showing NSBBs significantly reduced WBC and a reduction ≥15% was associated with a reduced risk of further decompensation and death.
Alvarado-Tapias E, Ardevol A, Garcia-Guix M, Montañés R, Pavel O, Cuyas B, et al. Short-term hemodynamic effects of β-blockers influence survival of patients with decompensated cirrhosis. J Hepatol. 2020;73(4):829–41. Retrospective study demonstrating that cardiac output can predict mortality in decompensated cirrhosis patients on NSBBs with a cut-off of 5L/min proposed.
Giannelli V, Roux O, Laouénan C, Manchon P, Ausloos F, Bachelet D, et al. Impact of cardiac function, refractory ascites and beta blockers on the outcome of patients with cirrhosis listed for liver transplantation. J Hepatol. 2020;72(3):463–71. Retrospective study showing patients on NSBBs with a left ventricular stroke work index <64.1g-m/m2 have increased transplant waiting list mortality.
Stefanescu H, Marasco G, Calès P, Fraquelli M, Rosselli M, Ganne-Carriè N, et al. A novel spleen-dedicated stiffness measurement by FibroScan® improves the screening of high-risk oesophageal varices. Liver Int. 2020;40(1):175–85.
Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51(3):475–82.
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426-1437.e9.
Madsen BS, Havelund T, Krag A. Targeting the gut–liver axis in cirrhosis: antibiotics and non-selective β-blockers. Adv Ther. 2013;30(7):659–70.
Jachs M, Hartl L, Simbrunner B, Bauer D, Paternostro R, Scheiner B, Schwabl P, Stättermayer AF, Pinter M, Eigenbauer E, Quehenberger P, Trauner M, Reiberger T, Mandorfer M. Decreasing von Willebrand factor levels upon nonselective beta blocker therapy indicate a decreased risk of further decompensation, acute-on-chronic liver failure, and death. Clin Gastroenterol Hepatol. 2022;20(6):1362–1373.e6. https://doi.org/10.1016/j.cgh.2021.07.012. Epub 2021 Jul 10. Retrospective study demonstrating that NSBBs reduce von Willebrand Factor. Individuals that demonstrate a significant reduction show decreased incidence of decompensation, ACLF, AKI and liver-related death.
Reiberger T. The value of liver and spleen stiffness for evaluation of portal hypertension in compensated Cirrhosis. Hepatol Commun. 2022;6(5):950–964. https://doi.org/10.1002/hep4.1855. Epub 2021 Dec 14.
Marasco G, Dajti E, Ravaioli F, Alemanni LV, Capuano F, Gjini K, et al. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hep Intl. 2020;14(5):850–7.
Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2021;116(4):723–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rajiv Jalan is the inventor of OPA, which has been patented by UCL and licensed to Mallinckrodt Pharma. He is also the founder of Yaqrit Discovery, a spin out company from University College London, Hepyx Limited and Cyberliver. He has research collaborations with Takeda and Yaqrit Discovery.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Portal Hypertension and Liver Transplantation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gananandan, K., Mookerjee, R. & Jalan, R. Use of Non-selective Beta blockers in Decompensated Cirrhosis and ACLF. Curr Hepatology Rep 21, 29–36 (2022). https://doi.org/10.1007/s11901-022-00584-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-022-00584-2