Abstract
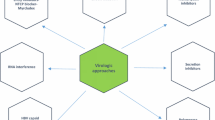
A number of immunomodulatory agents have been under study that facilitate a Th1 immune response, enhancing Tlymphocyte activity and stimulating interferon production. These include cytokines (interleukin [IL]-12, IL-10, IL-2), histamine, oral interferon-like molecules, ribavirin analogues, interferon gamma, thymosin alpha-1, amantadine, inosine-5′-monophosphate dehydrogenase inhibitors, tumor necrosis factor antagonists, and nonspecific hepatoprotectants (glycyrrhizin and caspase inhibitors). The current available data on these drugs are analyzed with an attempt to determine their usefulness as future hepatitis C virus treatments.
Similar content being viewed by others
References and Recommended Reading
Pockros P, Patel K, O’Brien CB: A multicenter study of recombinant human interleukin-12 for the treatment of chronic hepatitis C infection in patients with non-responsiveness to previous therapy. Hepatology 2003, 37:1368–1374.
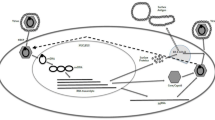
Thimme R, Bukh J, Spangenberg HC, et al.: Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A 2002, 99:15661–15668. An excellent discussion of viral determinants and CD4 and CD8 T-cell response associated with the clearance or persistence of HCV infection.
Erickson AL, Kimura Y, Igarashi S, et al.: The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 2001, 15:883–895.
Pockros PJ: Developments in the treatment of chronic hepatitis C. Expert Opin Investig Drugs 2002, 11:515–523. A thorough review of the monotherapy and combination therapy trials of pegylated interferons and of alternative strategies for drug development in the treatment of HCV.
McHutchison JG, Gordon SC, Schiff ER, et al.: Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998, 339:1485–1492.
Manns MP, McHutchison JG, Gordon SC, et al.: Peg-interferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001, 358:958–965.
Fried MW, Shiffman ML, Reddy KR, et al.: Peg-interferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002, 347:975–982.
Kamal SM, Fehr J, Roesler B, et al.: Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology 2002, 123:1070–1083.
Schlaak JF, Pitz T, Lohr HF, et al.: Interleukin 12 enhances deficient HCV-antigen-induced Th1-type immune response of peripheral blood mononuclear cells. J Med Virol 1998, 56:112–117.
Fan XG, Tang FQ, Yi H, et al.: Effect of IL-12 on T-cell immune responses in patients with chronic HCV infection. APMIS 2000, 108:531–538.
Zeuzem S, Hopf U, Carreno V, et al.: A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis C. Hepatology 1999, 29:1280–1286.
Moscarella S, Buzelli G, Romanelli RG, et al.: Interferon and thymosin combination therapy in naive patients with chronic hepatitis C: preliminary results. Liver 1998, 18:366–369.
Pardo M, Castillo I, Oliva H, et al.: A pilot study of recombinant interleukin-2 for treatment of chronic hepatitis C. Hepatology 1997, 26:1318–1321.
Lurie Y, Pakula R, Malnick S: Efficacy and safety of the combination of histamine dihydrochloride and interferon alpha-2b in a phase II trial in naive patients with chronic hepatitis C [abstract]. Hepatology 2001, 34:350A.
Rockey DC, Maher JJ, Jarnagin WR, et al.: Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology 1992, 16:776–784.
Nelson DR, Lauwers GY, Lau JY, Davis GL: Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology 2000, 118:655–660.
Nelson DR, Tu Z, Soldevila-Pico C, et al.: Long-term interleukin 10 therapy in chronic hepatitis C patients has a proven proviral and anti-inflammatory effect. Hepatology 2003, 38:859–868.
Serrate SA, Schulof RS, Leondaridis L, Goldstein AL: Modulation of human natural killer cell cytotoxic activity, lymphokine production, and interleukin 2 receptor expression by thymic hormones. J Immunol 1987, 139:2338–2343.
Moscarella S, Buzzelli G, Romanelli R, et al.: Interferon and thymosin combination therapy in naive patients with chronic hepatitis C. Liver 1998, 18:366–369.
Rasi G, Divigilio D, Mutchnick MG, et al.: Combination thymosin alpha 1 and lymphoblastoid interferon treatment in chronic hepatitis C. Gut 1996, 39:679–683.
Sherman K, Sjogren M, Creager R, et al.: Combination therapy with thymosin alpha1 and interferon for the treatment of chronic hepatitis C infection: a randomized, placebo-controlled double-blind trial. Hepatology 1998, 27:1128–1135.
Pockros PJ, Reindollar R, McHutchison JG, et al.: The safety and tolerability of daily infergen plus ribavirin in the treatment of naive chronic hepatitis C patients. J Viral Hepat 2003, 10:55–60.
Su AI, Pezaki JP, Wodicka L, et al.: Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A 2002, 99:15669–15674.
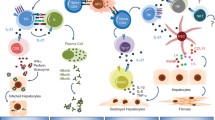
Guidotti LG, Chisari FV: Non-cytolytic control of viral infections by the innate and adaptive immune response. Ann Rev Immunol 2001, 19:65–91.
Tompkins WA: Immunomodulation and therapeutic effects of the oral use of interferon-alpha: mechanism of action. J Interferon Cytokine Res 1999, 19:817–828.
Goldstein D, Hertzog P, Tomkinson E, et al.: Administration of imiquimod, an interferon inducer, in asymptomatic human immunodeficiency virus-infected persons to determine safety and biologic response modification. J Infect Dis 1998, 178:858–861.
Pockros PJ, Tong M, Wright T, et al.: A phase IIa placebocontrolled, double-blind trial to determine the safety, tolerability, PK/PD of an oral interferon inducer, resiquimod, in chronic HCV. Gastroenterology 2003, 124(suppl 1):A-766.
Lau JYN, Tam RC, Liang TJ, Hong Z: Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology 2002, 35:1002–1009. The most comprehensive, well-written explanation of the likely mechanisms of action of RBV. The understanding of these potential mechanisms is critical to the development of similar immunomodulatory therapies.
McHutchison JG, Cheung R, Shiffman ML, et al.: A 4-week trial of VX 497 (an IMPDH inhibitor) combined with interferon in previously untreated patients with chronic hepatitis C. Hepatology 2001, 34(suppl):329A.
Afdhal N, Flamm S, Imperial JC, et al.: Analyses of 40 KDA peginterferon alfa-2a in combination with ribavirin, mycophenolate mofetil, amantadine or amantadine plus ribavirin in patients that relapsed or did not respond to Rebetron therapy: a report of two randomized, multicenter, efficacy and safety studies. Hepatology 2001, 34(suppl):243A.
Cornberg M, Hinrichsen H, Teuber G, et al.: Mycophenolate mofetil in combination with recombinant interferon alfa-2a in interferon-nonresponder patients with chronic hepatitis C. J Hepatol 2002, 37:843–847.
Fabris C, Del Forno M, Falleti E, et al.: Kinetics of serum soluble tumour necrosis factor receptor (TNF-R) type-I and type-II after a single interferon-alpha (IFN-alpha) injection in chronic hepatitis C. Clin Exp Immunol 1999, 117:556–560.
Tilg H, Vogel W, Dinarello CA: Interferon-alpha induces circulating tumor necrosis factor receptor p55 in humans. Blood 1995, 85:433–435.
Zein NN: A phase II randomized, double-blind, placebocontrolled study of tumor necrosis factor antagonist (Etanercept, Enbrel) as an adjuvant to interferon and ribavirin in naive patients with chronic hepatitis C. ETANERCEPT Study Group. Hepatology 2002, 36:304A.
Shi Z, Wakil AE, Rockey DC: Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A 1990, 94:10663–10668.
Rockey DC: Hepatic fibrogenesis and hepatitis C. Semin Gastrointest Dis 2000, 11:69–83.
Ziesche R, Hofbauer E, Wittmann K, et al.: A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999, 341:1264–1269. [Published erratum appears in N Engl J Med 2000, 342:524.]
Saez-Royuela F, Porres JC, Moreno A, et al.: High doses of recombinant alpha-interferon or gamma-interferon for chronic hepatitis C: a randomized, controlled trial. Hepatology 1991, 13:327–331.
Di Biscegli AM, Rustgi AK, Kassianides C, et al.: Therapy of chronic hepatitis B with recombinant human alpha and gamma interferon. Hepatology 1990, 11:266–270.
Kakumu S, Ishikawa T, Mizokami M, et al.: Treatment with human gamma interferon of chronic hepatitis B: comparaitve study with alpha interferon. J Med Virol 1991, 35:32–37.
Frese M, Schwarzle V, Barth K, et al.: Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 2002, 35:694–703.
Muir AJ, Sylvestre PB, Rockey DC: Interferon gamma-1 for the treatment of chronic hepatitis C infection. Gastroenterology 2003, 124(suppl):A-718.
Young KC, Lindsay KL, Lee KJ, et al.: Identification of a ribavirin-resistant NS5B mutation of hepatitis C virus during ribavirin monotherapy. Hepatology 2003, 38:869–878.
Davis GL, Wong JB, McHutchison JG, et al.: Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology 2003, 38:645–652.
Arora S, Lau D, Gish R, et al.: Phase I clinical studies of viramidine: a liver-targeting prodrug of ribavirin. Hepatology 2002, 36:356A.
Watson J: Prospects for hepatitis C virus therapeutics: levovirin and viramidine as improved derivatives of ribavirin. Curr Opin Investig Drugs 2002, 3:680–683.
Poupon RE, Bonnand AM, Queneau PE, et al.: Randomized trial of interferon-alpha plus ursodeoxycholic acid versus interferon plus placebo in patients with chronic hepatitis C resistant to interferon. Scand J Gastroenterol 2000, 35:642–649.
Lemon SM, Chisari FV, Lai MMC, et al.: The Nineteenth United States-Japan Joint Hepatitis Panel Meeting. Hepatology 1998, 28:881–887.
Arase Y, Ikeda K, Murashima N, et al.: The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997, 79:1494–1500.
Valentino K, Gutierrez M, Sanchez R, et al.: First clinical trial of IDN-6556: first anti-apoptotic caspace inhibitor improves liver function. Gastroenterology 2002, 122:A622.
Pockros P, Schiff E, Shiffman M, et al.: The first caspase inhibitor of apoptosis, IDN-6556, given orally lowers liver enzymes in HCV patients. Hepatology 2003, 38:1326.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pockros, P.J. Current status of immunomodulatory therapies in HCV infection. Curr hepatitis rep 3, 16–22 (2004). https://doi.org/10.1007/s11901-004-0004-y
Issue Date:
DOI: https://doi.org/10.1007/s11901-004-0004-y