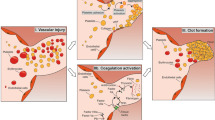
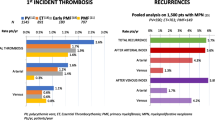
Abstract
Purpose of Review
Thrombosis remains a leading cause of morbidity and mortality in BCR/ABL negative myeloproliferative neoplasms (MPN). Circulating blood cells are both increased in quantity and qualitatively abnormal in MPN, resulting in an increased thrombotic risk. Herein, we review recently elucidated mechanisms of MPN thrombosis and discuss implications of drugs currently under investigation for MPN.
Recent Findings
Recent studies highlight that in JAK2V617F granulocytes and platelets, thrombo-inflammatory genes are upregulated. Furthermore, in JAK2V617F granulocytes, protein expression of integrin CD11b, tissue factor, and leukocyte alkaline phosphatase are all increased. Overall, myeloid cells, namely neutrophils, may contribute in several ways, such as through increased adhesion via β1 integrin binding to VCAM1, increased infiltration, and enhanced inducibility to extrude neutrophil extracellular traps. Non-myeloid inflammatory cells may also contribute via secretion of cytokines. With regard to red blood cells, number, rigidity, adhesion, and generation of microvesicles may lead to increased vascular resistance as well as increased cell-cell interactions that promote rolling and adhesion. Platelets may also contribute in a similar fashion. Lastly, the vasculature is also increasingly appreciated, as several studies have demonstrated increased endothelial expression of pro-coagulant and pro-adhesive proteins, such as von Willebrand factor or P-selectin in JAK2V617F endothelial cells.
Summary
With the advent of molecular diagnostics, MPN therapeutics are advancing beyond cytoreduction. Our increased understanding of pro-inflammatory and thrombotic pathophysiology in MPN provides a rational basis for evaluation of in-development MPN therapeutics to reduce thrombosis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Hultcrantz M, Björkholm M, Dickman PW, Landgren O, Derolf ÅR, Kristinsson SY, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Annals of Internal Medicine. 2018;168(5):317–25. Updated statistics on prevalence for thrombosis in MPN cohort.
Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120(25):4921–8.
Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667–79.
•• Cordua S, Kjaer L, Skov V, Pallisgaard N, Hasselbalch HC, Ellervik C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134(5):469–79. Prevalence of JAK2 clonal hematopoiesis in general population related to thrombosis.
Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595–600.
Grunwald MR, Stein BL, Boccia RV, Oh ST, Paranagama D, Parasuraman S, et al. Clinical and disease characteristics from REVEAL at time of enrollment (baseline): prospective observational study of patients with polycythemia vera in the United States. Clinical lymphoma, myeloma & leukemia. 2018.
Parasuraman SV, Shi N, Paranagama DC, Bonafede M. Health care costs and thromboembolic events in hydroxyurea-treated patients with polycythemia vera. J Manag Care Spec Pharm. 2018;24(1):47–55.
Lau WW, Hannah R, Green AR, Göttgens B. The JAK-STAT signaling pathway is differentially activated in CALR-positive compared with JAK2V617F-positive ET patients. Blood. 2015;125(10):1679–81.
Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123(22):e123–33.
Zhang Y, Zhou Y, Wang Y, Teng G, Li D, Wang Y, et al. Thrombosis among 1537 patients with JAK2(V617F) -mutated myeloproliferative neoplasms: risk factors and development of a predictive model. Cancer Med. 2020;9(6):2096–105.
Carobbio A, Ferrari A, Masciulli A, Ghirardi A, Barosi G, Barbui T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: a systematic review and meta-analysis. Blood advances. 2019;3(11):1729–37.
•• Gangaraju R, Song J, Kim SJ, Tashi T, Reeves BN, Sundar KM, et al. Thrombotic, inflammatory, and HIF-regulated genes and thrombosis risk in polycythemia vera and essential thrombocythemia. Blood advances. 2020;4(6):1115–30. Demonstrates increased thrombo-inflammatory gene signature in granulocytes and platelets.
Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653–8.
Song J, Sergueeva A, Miasnikova G, Kim SJ, Shah BN, Tashi T, et al. Phlebotomy-induced iron deficiency increases the expression of prothrombotic genes. Blood. 2020;136(Supplement 1):11–2.
Coucelo M, Caetano G, Sevivas T, Almeida Santos S, Fidalgo T, Bento C, et al. JAK2V617F allele burden is associated with thrombotic mechanisms activation in polycythemia vera and essential thrombocythemia patients. Int J Hematol. 2014;99(1):32–40.
Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96(13):4261–6.
Torregrosa JM, Ferrer-Marín F, Lozano ML, Moreno MJ, Martinez C, Anton AI, et al. Impaired leucocyte activation is underlining the lower thrombotic risk of essential thrombocythaemia patients with CALR mutations as compared with those with the JAK2 mutation. British journal of haematology. 2016;172(5):813–5.
Guo H, Chen X, Tian R, Chang J, Li J, Tan Y, et al. Frequencies, laboratory features, and granulocyte activation in Chinese patients with CALR-mutated myeloproliferative neoplasms. PLoS One. 2015;10(9):e0138250.
•• Edelmann B, Gupta N, Schnoder TM, Oelschlegel AM, Shahzad K, Goldschmidt J, et al. JAK2-V617F promotes venous thrombosis through beta1/beta2 integrin activation. J Clin Invest. 2018. Demonstrates importance of integrin activation and suggests role for anti-VCAM therapy.
Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ Res. 2018;123(11):e35–47.
•• Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Science translational medicine. 2018;10(436). Demonstrates role of neutrophil extracellular traps and a potential mechanisms for ruxolitinib mediated reduction of thrombosis.
Bogani C, Guglielmelli P, Antonioli E, Pancrazzi A, Bosi A, Vannucchi AM. B-, T-, and NK-cell lineage involvement in JAK2V617F-positive patients with idiopathic myelofibrosis. Haematologica. 2007;92(2):258–9.
Nishanth G, Wolleschak D, Fahldieck C, Fischer T, Mullally A, Perner F, et al. Gain of function in Jak2(V617F)-positive T-cells. Leukemia. 2017;31(4):1000–3.
Wang Y, Zuo X. Cytokines frequently implicated in myeloproliferative neoplasms. Cytokine: X. 2019;1(1):100005.
Stein BL, Martin K. From Budd-Chiari syndrome to acquired von Willebrand syndrome: thrombosis and bleeding complications in the myeloproliferative neoplasms. Blood. 2019;134(22):1902–11.
Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms. Journal of autoimmunity. 2017;85:58–63.
Lussana F, Carobbio A, Salmoiraghi S, Guglielmelli P, Vannucchi AM, Bottazzi B, et al. Driver mutations (JAK2V617F, MPLW515L/K or CALR), pentraxin-3 and C-reactive protein in essential thrombocythemia and polycythemia vera. J Hematol Oncol. 2017;10(1):54.
White J, Krishnamoorthy S, Gupta D, Lancelot M, Moore N, Sarnaik S, et al. VLA-4 blockade by natalizumab inhibits sickle reticulocyte and leucocyte adhesion during simulated blood flow. Br J Haematol. 2016;174(6):970–82.
White J, Lancelot M, Sarnaik S, Hines P. Increased erythrocyte adhesion to VCAM-1 during pulsatile flow: application of a microfluidic flow adhesion bioassay. Clin Hemorheol Microcirc. 2015;60(2):201–13.
Folsom AR, Wang W, Parikh R, Lutsey PL, Beckman JD, Cushman M, et al. Hematocrit and incidence of venous thromboembolism. Research and Practice in Thrombosis and Haemostasis. 2020;4(3):422–8.
Hultcrantz M, Modlitba A, Vasan SK, Sjolander A, Rostgaard K, Landgren O, et al. Hemoglobin concentration and risk of arterial and venous thrombosis in 1.5 million Swedish and Danish blood donors. Thromb Res. 2020;186:86–92.
Houghton DE, Koh I, Ellis A, Key NS, Douce DR, Howard G, et al. Hemoglobin levels and coronary heart disease risk by age, race, and sex in the reasons for geographic and racial differences in stroke study (REGARDS). Am J Hematol. 2020;95(3):258–66.
Wouters H, Mulder R, van Zeventer IA, Schuringa JJ, van der Klauw MM, van der Harst P, et al. Erythrocytosis in the general population: clinical characteristics and association with clonal hematopoiesis. Blood advances. 2020;4(24):6353–63.
Burger P, Kostova E, Bloem E, Hilarius-Stokman P, Meijer AB, van den Berg TK, et al. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. British Journal of Haematology. 2013;160(3):377–86.
Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, Renne T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10(7):1355–62.
Tong D, Yu M, Guo L, Li T, Li J, Novakovic VA, et al. Phosphatidylserine-exposing blood and endothelial cells contribute to the hypercoagulable state in essential thrombocythemia patients. Ann Hematol. 2018;97(4):605–16.
Tan X, Shi J, Fu Y, Gao C, Yang X, Li J, et al. Role of erythrocytes and platelets in the hypercoagulable status in polycythemia vera through phosphatidylserine exposure and microparticle generation. Thromb Haemost. 2013;109(6):1025–32.
Aswad MH, Kissová J, Rihova L, Zavrelova J, Ovesná P, Penka M. High level of circulating microparticles in patients with BCR/ABL negative myeloproliferative neoplasm—a pilot study. Klinicka onkologie : casopis Ceske a Slovenske onkologicke spolecnosti. 2019;32(2):109–16.
• Poisson J, Tanguy M, Davy H, Camara F, El Mdawar M-B, Kheloufi M, et al. Erythrocyte-derived microvesicles induce arterial spasms in JAK2V617F myeloproliferative neoplasm. The Journal of Clinical Investigation. 2020;130(5). Demonstrates increased RBC-microvesicles are associated with MPO-driven vascular activation.
Camus SM, De Moraes JA, Bonnin P, Abbyad P, Le Jeune S, Lionnet F, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125(24):3805–14.
Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10(7):1363–70.
Geddings JE, Hisada Y, Boulaftali Y, Getz TM, Whelihan M, Fuentes R, et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J Thromb Haemost. 2015.
Whelihan MF, Lim MY, Key NS. Red blood cells and thrombin generation in sickle cell disease. Thromb Res. 2014;133(Suppl 1):S52–3.
Gautier EF, Leduc M, Cochet S, Bailly K, Lacombe C, Mohandas N, et al. Absolute proteome quantification of highly purified populations of circulating reticulocytes and mature erythrocytes. Blood advances. 2018;2(20):2646–57.
Brusson M, De Grandis M, Cochet S, Bigot S, Marin M, Leduc M, et al. Impact of hydroxycarbamide and interferon-alpha on red cell adhesion and membrane protein expression in polycythemia vera. Haematologica. 2018;103(6):972–81.
Hebbel RP, Key NS. Microparticles in sickle cell anaemia: promise and pitfalls. Br J Haematol. 2016;174:16–29.
Nader E, Skinner S, Romana M, Fort R, Lemonne N, Guillot N, et al. Blood rheology: key parameters, impact on blood flow, role in sickle cell disease and effects of exercise. Front Physiol. 2019;10:1329.
Chien S, Usami S, Dellenback RJ, Gregersen MI, Nanninga LB, Guest MM. Blood viscosity: influence of erythrocyte aggregation. Science. 1967;157(3790):829–31.
Walton BL, Lehmann M, Skorczewski T, Holle LA, Beckman JD, Cribb JA, et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129(18):2537–46.
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33.
Alvarez-Larrán A, Pereira A, Cervantes F, Arellano-Rodrigo E, Hernández-Boluda JC, Ferrer-Marín F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119(6):1363–9.
Barbui T, Vannucchi AM, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5(11):e369.
Campbell PJ, MacLean C, Beer PA, Buck G, Wheatley K, Kiladjian JJ, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120(7):1409–11.
Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857–9.
Tefferi A, Szuber N, Pardanani A, Hanson CA, Vannucchi AM, Barbui T, et al. Extreme thrombocytosis in low-risk essential thrombocythemia: retrospective review of vascular events and treatment strategies. American journal of hematology. 2021.
Budde U, Scharf RE, Franke P, Hartmann-Budde K, Dent J, Ruggeri ZM. Elevated platelet count as a cause of abnormal von Willebrand factor multimer distribution in plasma. Blood. 1993;82(6):1749–57.
Michiels JJ, Berneman Z, Schroyens W, Finazzi G, Budde U, van Vliet HH. The paradox of platelet activation and impaired function: platelet-von Willebrand factor interactions, and the etiology of thrombotic and hemorrhagic manifestations in essential thrombocythemia and polycythemia vera. Semin Thromb Hemost. 2006;32(6):589–604.
Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, et al. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia. 2012;26(4):716–9.
Hobbs CM, Manning H, Bennett C, Vasquez L, Severin S, Brain L, et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013;122(23):3787–97.
Lamrani L, Lacout C, Ollivier V, Denis CV, Gardiner E. Ho Tin Noe B, et al. Hemostatic disorders in a JAK2V617F-driven mouse model of myeloproliferative neoplasm. Blood. 2014;124(7):1136–45.
• Matsuura S, Thompson CR, Belghasem ME, Bekendam RH, Piasecki A, Leiva O, et al. Platelet dysfunction and thrombosis in JAK2(V617F)-mutated primary myelofibrotic mice. Arterioscler Thromb Vasc Biol. 2020;40(10):e262–e72. Demonstrates reduced platelet granule formation in JAK2V617F mouse MF model.
Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Experimental hematology. 2005;33(5):523–30.
Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91(2):169–75.
Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35(5):702–11.
Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, Colomer D, Villamor N, Bellosillo B, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2009;84(2):102–8.
Cunin P, Bouslama R, Machlus KR, Martínez-Bonet M, Lee PY, Wactor A, et al. Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. Elife. 2019;8.
Stein BL, McMahon B, Weiss I, Kwaan HC. Tissue-factor bearing microparticles and thrombotic risk in the myeloproliferative neoplasms. Blood. 2012;120(21):1145.
Zhang W, Qi J, Zhao S, Shen W, Dai L, Han W, et al. Clinical significance of circulating microparticles in Ph(-) myeloproliferative neoplasms. Oncology letters. 2017;14(2):2531–6.
Charpentier A, Lebreton A, Rauch A, Bauters A, Trillot N, Nibourel O, et al. Microparticle phenotypes are associated with driver mutations and distinct thrombotic risks in essential thrombocythemia. Haematologica. 2016;101(9):e365–8.
Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113(21):5246–9.
Rosti V, Villani L, Riboni R, Poletto V, Bonetti E, Tozzi L, et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2 V617F mutation. Blood. 2013;121(2):360–8.
Helman R, Pereira WO, Marti LC, Campregher PV, Puga RD, Hamerschlak N, et al. Granulocyte whole exome sequencing and endothelial JAK2V617F in patients with JAK2V617F positive Budd-Chiari Syndrome without myeloproliferative neoplasm. Br J Haematol. 2018;180(3):443–5.
Guadall A, Lesteven E, Letort G, Awan Toor S, Delord M, Pognant D, et al. Endothelial cells harbouring the JAK2V617F mutation display pro-adherent and pro-thrombotic features. Thromb Haemost. 2018.
•• Guy A, Gourdou-Latyszenok V, Le Lay N, Peghaire C, Kilani B, Dias JV, et al. Vascular endothelial cell expression of JAK2(V617F) is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica. 2019;104(1):70–81. Demonstrates increased P-selectin expression and suggest potential mechanisms for hydroxyurea as well as crizanlizumab.
Castiglione M, Jiang Y-P, Mazzeo C, Lee S, Chen J-S, Kaushansky K, et al. Endothelial JAK2V617F mutation leads to thrombosis, vasculopathy, and cardiomyopathy in a murine model of myeloproliferative neoplasm. Journal of Thrombosis and Haemostasis. n/a(n/a).
Sano S, Wang Y, Yura Y, Sano M, Oshima K, Yang Y, et al. JAK2 (V617F) -mediated clonal hematopoiesis accelerates pathological remodeling in murine heart failure. JACC Basic to translational science. 2019;4(6):684–97.
Tripodo C, Burocchi A, Piccaluga PP, Chiodoni C, Portararo P, Cappetti B, et al. Persistent immune stimulation exacerbates genetically driven myeloproliferative disorders via stromal remodeling. Cancer Res. 2017;77(13):3685–99.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(12):1599–613.
Cokic VP, Beleslin-Cokic BB, Tomic M, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood. 2006;108(1):184–91.
El Nemer W, De Grandis M, Brusson M. Abnormal adhesion of red blood cells in polycythemia vera: a prothrombotic effect? Thromb Res. 2014;133(Suppl 2):S107–11.
Vainchenker W, Leroy E, Gilles L, Marty C, Plo I, Constantinescu SN. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res. 2018;7:82.
Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–35.
Mullally A, Hood J, Harrison C, Mesa R. Fedratinib in myelofibrosis. Blood advances. 2020;4(8):1792–800.
Betts BC, Bastian D, Iamsawat S, Nguyen H, Heinrichs JL, Wu Y, et al. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci U S A. 2018;115(7):1582–7.
Samuelson BT, Vesely SK, Chai-Adisaksopha C, Scott BL, Crowther M, Garcia D. The impact of ruxolitinib on thrombosis in patients with polycythemia vera and myelofibrosis: a meta-analysis. Blood Coagul Fibrinolysis. 2016;27(6):648–52.
•• Masciulli A, Ferrari A, Carobbio A, Ghirardi A, Barbui T. Ruxolitinib for the prevention of thrombosis in polycythemia vera: a systematic review and meta-analysis. Blood advances. 2020;4(2):380–6. Updated meta-analysis of thrombosis rates in ruxolitinib clinical trials.
Keohane C, McLornan DP, Sanchez K, Connor C, Radia D, Harrison CN. The effects of JAK inhibitor therapy upon novel markers of thrombosis in myeloproliferative neoplasms. Haematologica. 2015;100(9):e348–e50.
• Singer JW, Al-Fayoumi S, Taylor J, Velichko S, O’Mahony A. Comparative phenotypic profiling of the JAK2 inhibitors ruxolitinib, fedratinib, momelotinib, and pacritinib reveals distinct mechanistic signatures. PLOS ONE. 2019;14(9):e0222944. Unbiased assessment of cell activation patterns in response to JAK2 inhibitors.
Curran SA, Shyer JA, St Angelo ET, Talbot LR, Sharma S, Chung DJ, et al. Human dendritic cells mitigate NK-cell dysfunction mediated by nonselective JAK1/2 blockade. Cancer immunology research. 2017;5(1):52–60.
Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nature reviews Drug discovery. 2016;15(3):173–83.
Kleppe M, Koche R, Zou L, van Galen P, Hill CE, Dong L, et al. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer cell. 2018;33(1):29-43.e7.
Wang N, Wu R, Tang D, Kang R. The BET family in immunity and disease. Signal Transduct Target Ther. 2021;6(1):23.
Zhou Z, Li X, Liu Z, Huang L, Yao Y, Li L, et al. A bromodomain-containing protein 4 (BRD4) inhibitor suppresses angiogenesis by regulating AP-1 expression. Front Pharmacol. 2020;11:1043.
Parry GCN, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(5):612–21.
Chifotides HT, Bose P, Verstovsek S. Givinostat: an emerging treatment for polycythemia vera. Expert Opin Investig Drugs. 2020;29(6):525–36.
Amaru Calzada A, Todoerti K, Donadoni L, Pellicioli A, Tuana G, Gatta R, et al. The HDAC inhibitor Givinostat modulates the hematopoietic transcription factors NFE2 and C-MYB in JAK2(V617F) myeloproliferative neoplasm cells. Exp Hematol. 2012;40(8):634-45.e10.
Yue L, Sharma V, Horvat NP, Akuffo AA, Beatty MS, Murdun C, et al. HDAC11 deficiency disrupts oncogene-induced hematopoiesis in myeloproliferative neoplasms. Blood. 2020;135(3):191–207.
Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009;114(24):5024–33.
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280(29):26729–34.
Weigert O, Lane AA, Bird L, Kopp N, Chapuy B, van Bodegom D, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med. 2012;209(2):259–73.
Marubayashi S, Koppikar P, Taldone T, Abdel-Wahab O, West N, Bhagwat N, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010;120(10):3578–93.
Rambaldi A, Iurlo A, Vannucchi AM, Noble R, von Bubnoff N, Guarini A, et al. Safety and efficacy of the maximum tolerated dose of givinostat in polycythemia vera: a two-part Phase Ib/II study. Leukemia. 2020;34(8):2234–7.
Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P, et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010;150(4):446–55.
Amaru Calzada A, Pedrini O, Finazzi G, Leoni F, Mascagni P, Introna M, et al. Givinostat and hydroxyurea synergize in vitro to induce apoptosis of cells from JAK2(V617F) myeloproliferative neoplasm patients. Exp Hematol. 2013;41(3):253-60.e2.
Leoni F, Fossati G, Lewis EC, Lee JK, Porro G, Pagani P, et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med. 2005;11(1-12):1–15.
Golay J, Cuppini L, Leoni F, Micò C, Barbui V, Domenghini M, et al. The histone deacetylase inhibitor ITF2357 has anti-leukemic activity in vitro and in vivo and inhibits IL-6 and VEGF production by stromal cells. Leukemia. 2007;21(9):1892–900.
Furlan A, Monzani V, Reznikov LL, Leoni F, Fossati G, Modena D, et al. Pharmacokinetics, safety and inducible cytokine responses during a phase 1 trial of the oral histone deacetylase inhibitor ITF2357 (givinostat). Mol Med. 2011;17(5-6):353–62.
Hebbel RP, Vercellotti GM, Pace BS, Solovey AN, Kollander R, Abanonu CF, et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood. 2010;115(12):2483–90.
Yao X, Chen W, Liu J, Liu H, Zhan JY, Guan S, et al. Deep vein thrombosis is modulated by inflammation regulated via sirtuin 1/NF-κB signalling pathway in a rat model. Thromb Haemost. 2019;119(3):421–30.
Finazzi G, Iurlo A, Martino B, Carli G, Guarini A, Noble R, et al. A Long-term safety and efficacy study of givinostat in patients with polycythemia vera: the first 4 years of treatment. Blood. 2017;130(Supplement 1):1648.
Tremblay D, Mascarenhas J. Novel therapies in polycythemia vera. Curr Hematol Malig Rep. 2020;15(2):133–40.
Ginzburg YZ, Feola M, Zimran E, Varkonyi J, Ganz T, Hoffman R. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32:2105–16.
Casu C, Nemeth E, Rivella S. Hepcidin agonists as therapeutic tools. Blood. 2018;131(16):1790–4.
Ellingsen TS, Lappegard J, Ueland T, Aukrust P, Braekkan SK, Hansen JB. Plasma hepcidin is associated with future risk of venous thromboembolism. Blood advances. 2018;2(11):1191–7.
Tang X, Fang M, Cheng R, Zhang Z, Wang Y, Shen C, et al. Iron-deficiency and estrogen are associated with ischemic stroke by up-regulating transferrin to induce hypercoagulability. Circ Res. 2020;127(5):651–63.
Jimenez K, Leitner F, Leitner A, Scharbert G, Schwabl P, Kramer AM, et al. Iron deficiency induced thrombocytosis increases thrombotic tendency in rats. Haematologica. 2020.
Casu C, Oikonomidou PR, Chen H, Nandi V, Ginzburg Y, Prasad P, et al. Minihepcidin peptides as disease modifiers in mice affected by β-thalassemia and polycythemia vera. Blood. 2016;128(2):265–76.
Kremyanskaya M, Ginzburg Y, Kuykendall AT, Yacoub A, Yang J, Gupta SK, et al. PTG-300 eliminates the need for therapeutic phlebotomy in both low and high-risk polycythemia vera patients. Blood. 2020;136(Supplement 1):33–5.
Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107(7):2736–44.
Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P–selectin–dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95(10):3146–52.
Etheridge SL, Roh ME, Cosgrove ME, Sangkhae V, Fox NE, Chen J, et al. JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Natl Acad Sci U S A. 2014;111(6):2295–300.
Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–39.
Funding
Funding: Dr. Beckman received funding from Bayer and Novartis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joan Beckman has received grants from Bayer and Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part on the Topical Collection on Myeloproliferative Neoplasms
Rights and permissions
About this article
Cite this article
Reeves, B.N., Beckman, J.D. Novel Pathophysiological Mechanisms of Thrombosis in Myeloproliferative Neoplasms. Curr Hematol Malig Rep 16, 304–313 (2021). https://doi.org/10.1007/s11899-021-00630-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00630-8