Abstract
Purpose of Review
Extranodal NK/T cell lymphoma (ENKL), nasal type, is a highly aggressive lymphoma which used to show a poor clinical outcome. Expression of P-glycoprotein on lymphoma cells of ENKL is a major reason for the refractoriness to conventional chemotherapy containing anthracycline. However, recent innovative approaches have improved the outcome and prognosis of ENKL. The purpose of this review is to summarize the proceedings of treatment.
Recent Findings
Concurrent chemoradiotherapy containing platinum and several drugs including L-asparaginase, methotrexate, and alkylators shows excellent outcomes for the limited-stage ENKL. SMILE (steroid, methotrexate, ifosfamide, L-asparaginase, and etoposide) or other L-asparaginase-containing therapy is promising for advanced-stage ENKL, followed by either autologous or allogeneic hematopoietic stem cell transplantation. Anti-PD-1 or other immunological checkpoint inhibitors are recently reported to be effective for relapsed/refractory ENKL thought to be due to EBV-driven upregulation of PD-L1 expression.
Summary
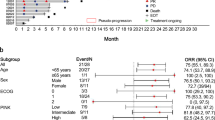
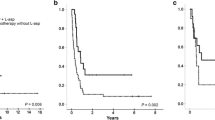
The prognosis of ENKL is therefore improving by the introduction of these strategies. The 5-year overall survival (OS) rate of limited stage was 63.2% [95% confidence interval (CI), 55.3 to 70.0%] before 2010, but was 79.4% (95% CI, 66.9 to 87.6%) in 2010 or after. However, there still exists a room for improvement, particularly for advanced-stage patients. The 2-year OS of advanced ENKL was 30.3% (95% CI, 19.5 to 41.7%) before 2010, but was 40.5% (95% CI, 24.8 to 55.8%) in 2010 or after. Optimal treatment scheme should further be explored.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chan JKC, Quintanilla-Martinez L, Ferry JA. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of Tumours of Haematopoietic and lymphoid tissues. Lyon, France: IARC; 2017. p. 368–71.
Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, et al. Prognostic factors for mature natural killer (NK)-cell neoplasms: aggressive NK-cell leukemia and extranodal NK-cell lymphoma, nasal-type. Ann Oncol. 2010;21(5):1032–40. https://doi.org/10.1093/annonc/mdp418.
Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389–400. https://doi.org/10.1016/S1470-2045(15)00533-1.
Cheung MMC, Chan JKC, Lau WH, et al. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998;16(1):70–7. https://doi.org/10.1200/JCO.1998.16.1.70.
Kim GE, Koom WS, Yang WI, Lee SW, Keum KC, Lee CG, et al. Clinical relevance of three subtypes of primary sinonasal lymphoma characterized by immunophenotypic analysis. Head Neck. 2004;26(7):584–93. https://doi.org/10.1002/hed.20015.
Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36(4):481–99. https://doi.org/10.1097/PAS.0b013e31824433d8.
Sakajiri S, Kawamata N, Egashira M, Mori K, Oshimi K. Molecular analysis of tumor suppressor genes, Rb, p53, p16INK4A, p15INK4B and p14ARF in natural killer cell neoplasms. Jpn J Cancer Res. 2001;92(10):1048–56. https://doi.org/10.1111/j.1349-7006.2001.tb01059.x.
Nakashima Y, Tagawa H, Suzuki R, Karnan S, Karube K, Ohshima K, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal NK/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. 2005;44(3):247–55. https://doi.org/10.1002/gcc.20245.
Berti E, Recalcati S, Girgenti V, Fanoni D, Venegoni L, Vezzoli P. Cutaneous extranodal NK/T-cell lymphoma: a clinicopathologic study of 5 patients with array-based comparative genomic hybridization. Blood. 2010;116(2):165–70. https://doi.org/10.1182/blood-2009-11-252957.
Ahn HK, Suh C, Chuang SS, Suzumiya J, Ko YH, Kim SJ, et al. Extranodal natural killer/T cell lymphoma from skin or soft tissue: suggestion of treatment from multinational retrospective analysis. Ann Oncol. 2012;23(10):2703–7. https://doi.org/10.1093/annonc/mds096.
Kim SJ, Jung HA, Chuang SS, Hong H, Guo CC, Cao J, et al. Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: analysis of clinical features and outcomes from the Asia lymphoma study group. J Hematol Oncol. 2013;6(1):86. https://doi.org/10.1186/1756-8722-6-86.
Suzuki R, Suzumiya J, Oshimi K. Differences between nasal and extra-nasal NK/T-cell lymphoma. Blood. 2009;113(24):6260–1. https://doi.org/10.1182/blood-2009-03-211011.
Egashira M, Kawamata N, Sugimoto K, Kaneko T, Oshimi K. P-glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of P-glycoprotein function by cyclosporin A and its analogue, PSC833. Blood. 1999;93(2):599–606.
Kim WS, Song SY, Ahn YC, Ko YH, Baek CH, Kim DY, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001;12(3):349–52. https://doi.org/10.1023/A:1011144911781.
Kim SJ, Kim BS, Choi CW, Seo HY, Seol HR, Sung HJ, et al. Treatment outcome of front-line systemic chemotherapy for localized extranodal NK/T cell lymphoma in nasal and upper aerodigestive tract. Leuk Lymphoma. 2006;47(7):1265–73. https://doi.org/10.1080/10428190600565651.
Li YX, Coucke PA, Li JY, Gu DZ, Liu XF, Zhou LQ, et al. Primary non-Hodgkin's lymphoma of the nasal cavity: prognostic significance of paranasal extension and the role of radiotherapy and chemotherapy. Cancer. 1998;83(3):449, 1998–56. https://doi.org/10.1002/(SICI)1097-0142(19980801)83:3<449::AID-CNCR13>3.0.CO;2-W.
Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24(1):181–9. https://doi.org/10.1200/JCO.2005.03.2573.
You JY, Chi KH, Yang MH, Chen CC, Ho CH, Chau WK, et al. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: a single institute survey in Taiwan. Ann Oncol. 2004;15(4):618–25. https://doi.org/10.1093/annonc/mdh143.
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan clinical oncology group study JCOG0211. J Clin Oncol. 2009;27(33):5594–600. https://doi.org/10.1200/JCO.2009.23.8295.
Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027–32. https://doi.org/10.1200/JCO.2009.23.8592.
Kim SJ, Yang DH, Kim JS, Kwak JY, Eom HS, Hong DS, et al. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol. 2014;93(11):1895–901. https://doi.org/10.1007/s00277-014-2137-6.
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, et al. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan clinical oncology group study JCOG0211. J Clin Oncol. 2012;30(32):4044–6. https://doi.org/10.1200/JCO.2012.45.6541.
•• Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma who were diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol. 2017;35(1):32–9. The NKEA study showed that prognosis of limited-stage ENKL has improved in the daily practice. https://doi.org/10.1200/JCO.2016.68.1619.
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia lymphoma study group. Blood. 2012;120(15):2973–80. https://doi.org/10.1182/blood-2012-05-431460.
Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol. 2017.
Nagafuji K, Fujisaki T, Arima F, Ohshima K. L-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol. 2001;74(4):447–50. https://doi.org/10.1007/BF02982090.
Obama K, Tara M, Niina K. L-asparaginase-based induction therapy for advanced extranodal NK/T-cell lymphoma. Int J Hematol. 2003;78(3):248–50. https://doi.org/10.1007/BF02983802.
Matsumoto Y, Nomura K, Kanda-Akano Y, Fujita Y, Nakao M, Ueda K, et al. Successful treatment with Erwinia L-asparaginase for recurrent natural killer/T cell lymphoma. Leuk Lymphoma. 2003;44(5):879–82. https://doi.org/10.1080/1042819031000067873.
Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005;130(6):860–8. https://doi.org/10.1111/j.1365-2141.2005.05694.x.
Suzuki R. Treatment of advanced extranodal NK/T cell lymphoma, nasal-type and aggressive NK-cell leukemia. Int J Hematol. 2010;92(5):697–701. https://doi.org/10.1007/s12185-010-0726-2.
Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly-diagnosed stage IV, relapsed or refractory extranodal NK/T-cell lymphoma, nasal type: the NK-cell tumor study group (NKTSG) study. J Clin Oncol. 2011;29(33):4410–6. https://doi.org/10.1200/JCO.2011.35.6287.
Suzuki R, Kwong Y, Maeda Y, et al. 5-year follow-up of the SMILE phase II study for newly diagnosed stage IV, relapsed or refractory extranodal NK/T-cell lymphoma, nasal type. Hematol Oncol. 2015;33(Supple. S1):140. [Abstract #075]
Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase II study. Blood. 2011;117(6):1834–9. https://doi.org/10.1182/blood-2010-09-307454.
Jaccard A, Suerez F, Delmer A, et al. A prospective phase II trial of an L-asparaginase containing regimen in extranodal NK/T-cell lymphoma. Hematol Oncol. 2013;31(Supple. S1):129. [Abstract #099]
Suzuki R. Leukemia and lymphoma of natural killer cells. J Clin Exp Hematop. 2005;45(2):51–70. https://doi.org/10.3960/jslrt.45.51.
Au WY, Lie AK, Liang R, Kwong YL, Yau CC, Cheung MM, et al. Autologous stem cell transplantation for nasal NK/T-cell lymphoma: a progress report on its value. Ann Oncol. 2003;14(11):1673–6. https://doi.org/10.1093/annonc/mdg458.
Suzuki R, Suzumiya J, Nakamura S, et al. Hematopoietic stem cell transplantation for natural killer-cell lineage neoplasms. Bone Marrow Transplant. 2006;37(4):425–31. https://doi.org/10.1038/sj.bmt.1705244.
Kim HJ, Bang SM, Lee J, Kwon HC, Suh C, Kim HJ, et al. High-dose chemotherapy with autologous stem cell transplantation in extranodal NK/T-cell lymphoma: a retrospective comparison with non-transplantation cases. Bone Marrow Transplant. 2006;37(9):819–24. https://doi.org/10.1038/sj.bmt.1705349.
Yhim HY, Kim JS, Mun YC, Moon JH, Chae YS, Park Y, et al. Clinical outcomes and prognostic factors of up-front autologous stem cell transplantation in patients with extranodal natural killer/T cell lymphoma. Biol Blood Marrow Transplant. 2015;21(9):1597–604. https://doi.org/10.1016/j.bbmt.2015.05.003.
Lee J, Au WY, Park MJ, Suzumiya J, Nakamura S, Kameoka JI, et al. Autologous hematopoietic stem cell transplantation in extranodal NK/T-cell lymphoma: a multinational, multicenter, matched controlled study. Biol Blood Marrow Transplant. 2008;14(12):1356–64. https://doi.org/10.1016/j.bbmt.2008.09.014.
• Kharfan-Dabaja MA, Kumar A, Ayala E, Hamadani M, Reimer P, Gisselbrecht C, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T-cell and NK/T-cell lymphomas: an international collaborative effort on behalf of the Guidelines Committee of the American Society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2017;23(11):1826–38. The ASBMT recommendation summarized the opinion of lymphoma experts for the HSCT in ENKL. https://doi.org/10.1016/j.bbmt.2017.07.027.
Murashige N, Kami M, Kishi Y, Kim SW, Takeuchi M, Matsue K, et al. Allogeneic haematopoietic stem cell transplantation as a promising treatment for natural killer-cell neoplasms. Br J Haematol. 2005;130(4):561–7. https://doi.org/10.1111/j.1365-2141.2005.05651.x.
Kanate AS, DiGilio A, Ahn KW, et al. Allogeneic haematopoietic cell transplantation for extranodal natural killer/T-cell lymphoma, nasal type: a CIBMTR analysis. Br J Haematol. 2017.
Suzuki R, Kako S, Hyo R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for extranodal NK/T-cell lymphoma, nasal-type: analysis of the Japan Society for Hematopoietic Cell Transplantation (JSHCT) lymphoma working group. Blood. 2011;118:503a. [Abstract #503]
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. https://doi.org/10.1097/COC.0000000000000239.
Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O'Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8. https://doi.org/10.1158/1078-0432.CCR-11-1942.
Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MGM, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–73. https://doi.org/10.1158/1078-0432.CCR-13-0855.
•• Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood. 2017;129(17):2437–42. The checkpoint inhibitor antibody was dramatically effective for relapsed/refractory ENKL. https://doi.org/10.1182/blood-2016-12-756841.
Chan TSY, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ritsuro Suzuki reports personal fees from Chugai Pharmaceutical, personal fees from Kyowa-Hakko Kirin Pharmaceutical, personal fees from Takeda Pharmaceutical, personal fees from Meiji Seika Pharma, personal fees from Bristol-Meyers Squib, personal fees from Celgene Inc., personal fees from MSD Inc., personal fees from Sanofi Inc., personal fees from Gilead Sciences, personal fees from Mundi Pharma, personal fees from Jazz Pharma, personal fees from Mochida Pharma, personal fees from Novartis Pharma, personal fees from Sysmex Inc., and personal fees from Sumitomo Dainippon.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on T Cell and Other Lymphoproliferative Malignancies
Rights and permissions
About this article
Cite this article
Suzuki, R. NK/T Cell Lymphoma: Updates in Therapy. Curr Hematol Malig Rep 13, 7–12 (2018). https://doi.org/10.1007/s11899-018-0430-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-018-0430-5