Abstract
Purpose of Review
Colorectal cancer (CRC) screening is recommended to reduce CRC mortality. This review outlines key factors to consider when recommending screening, including disease burden, screening benefits and harms, and remaining knowledge gaps.
Recent Findings
In response to increasing rates of CRC incidence among younger (age < 50 years) adults, the American Cancer Society published guidelines in May 2018 recommending average-risk CRC screening beginning at age 45 (vs. 50) years. Rates of young-onset CRC have increased in the USA since the early 1990s. However, there is very little empirical evidence of screening effectiveness in younger adults, and few studies have reported harms of routine screening in this age group. Further, we know little about the natural history of CRC in younger adults.
Summary
Uncertainty surrounding the efficacy of CRC screening in younger adults suggests the benefits may be small. Precision cancer screening—or modified screening regimens based on risk—may improve the balance of screening benefits and harms beyond conventional age-based strategies.
Similar content being viewed by others
Introduction
Since the late 1980s [1, 2], colorectal cancer (CRC) screening with stool-based tests, flexible sigmoidoscopy, or colonoscopy has been recommended to reduce CRC mortality [3, 4••]. Population-wide screening has led to dramatic declines in both incidence and mortality [5], and CRC screening has been touted as one of the most effective preventive health services [6, 7]. Incidence and mortality rates have decreased by more than 30% in the USA since 1985, with particularly steep declines among those over age 65 years [8].
Unlike screening for other cancers, where questions linger concerning if, when, how, and how often to screen, historically, there has been consensus across professional organizations that CRC screening should start at age 50 years for average risk adults (Table 1). A number of randomized controlled trials and observational studies with mortality endpoints provide strong evidence supporting the effectiveness of guaiac-based fecal occult blood tests (gFOBT) [17, 18], sigmoidoscopy [19,20,21], and colonoscopy [22, 23]. More recent advances in fecal immunochemical tests (FIT) [24], as well as the availability of CT colonography and FIT-DNA, provide several additional options for screening, all regarded as equally effective [25]. Others have demonstrated efficacy of multi-component interventions [26] to increase patient adherence to screening, including mailed outreach, patient reminders, and reduced structural barriers. Collectively, the field has made enormous progress in understanding CRC biology and screening methods, offering continued support for starting average-risk screening at age 50 years.
In May 2018, the American Cancer Society (ACS) published updated guidelines recommending average-risk CRC screening beginning at age 45 years [16••]. The ACS commissioned these guidelines in response to increasing rates of CRC incidence among younger (age < 50 years) adults [27••]. Yet, there is very little empirical evidence of screening effectiveness in those under age 50 years. Nearly all randomized trials of screening efficacy are limited to age ≥ 50 years, and few or no studies have reported harms of routine screening among 40-year olds. Given the lack of evidence, ACS guidelines rely on simulation models and assumptions [28, 29••], extrapolating evidence of screening efficacy and adverse events from older populations. As such, the new recommendation to initiate CRC screening at age 45 years is qualified—carrying some uncertainty about the balance of screening benefits and harms in this younger age group.
Screening for any disease in the general population requires thoughtful consideration of disease burden, as well as the benefits and harms of screening. New guidelines have led to an impassioned debate about when to initiate CRC screening among adults at average risk, and many have called for more evidence on screening benefits and harms among 45–49-year olds [30•]. Thought leaders in the field have raised concerns about the implications of screening an additional 22 million adults—worsening disparities [31•], insufficient endoscopic capacity [32•], and cost to the healthcare system [33•, 34•]. Differences in the ACS and prior guidelines may also cause confusion among patients about what to do in the face of disagreement. Most average-risk adults undergoing CRC screening, even those with abnormal findings, will never develop the disease. The lifetime risk is about 1 in 22 (4.5%), and decisions of who and how often to screen should consider the consequences for the remaining 95% of the population who will never develop cancer. Conflicting guidelines and the surrounding debate raise two related questions:
-
1)
In adults ages 45–49 and 50–75 years, asymptomatic, and at average risk of CRC, what is the balance of benefits and harms in those offered screening compared with those not offered screening?
-
2)
What is the effect of the age at screening initiation on the balance of benefits and harms?
This review outlines key factors to consider when addressing these questions, including disease burden and natural history, screening benefits (i.e., effectiveness), screening harms (i.e., adverse effects), and remaining knowledge gaps. Table 2 also summarizes these key factors.
Disease Burden
Epidemiology of Young-Onset CRC
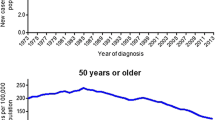
CRC incidence and mortality trends have evolved strikingly in recent decades. Despite large declines in older populations (Fig. 1), the incidence of CRC has nearly doubled among younger adults since the early 1990s [27••]. Incidence rates have risen rapidly among those ages 20–49 years in the USA, from 8.6 per 100,000 in 1992 to 12.5 per 100,000 in 2015, with the largest absolute increases among 40-year olds (from 18.2 to 26.5 per 100,000) [8]. Mortality rates have only increased slightly during the same period, ranging from 7.2 to 8.3 per 100,000 among the 45–49-year age group. Similar increases in incidence and mortality have occurred across the globe—from France [48] to Canada [49] to Australia [50]. Despite overall population trends in aging, by 2030, about 11% of colon cancers and 23% of rectal cancers in the USA will occur in adults younger than age 50 years [51].
Unrecognized hereditary syndromes and family history of CRC or advanced adenomas may explain a substantial proportion of young-onset CRC. About 15% of young adults diagnosed with CRC carry mutations in genes associated with Lynch syndrome or polyposis [52••, 53••]. Another 5% have mutations in genes not traditionally associated with CRC (e.g., BRCA, ATM), and 15% report a family history of CRC but no known hereditary syndrome. Many of these patients are eligible for earlier screening under existing clinical guidelines—most organizations recommend patients with a first-degree relative with CRC or advanced adenoma start screening at age 40 or 10 years younger than the earliest family diagnosis [11]. Other guidelines make specific recommendations for screening in the setting of hereditary syndromes [13].
Mechanisms contributing to the other 55% of young-onset CRC cases remain largely unknown. The rise in incidence has occurred more rapidly than expected if it were entirely due to genetics, and environmental risk factors likely play a role [54]. And, for those with hereditary syndromes or family history, environmental risk factors may modify penetrance and contribute to younger age at onset. Many have hypothesized that obesity [55] and diet account for the majority of sporadic young-onset CRC, but these risk factors alone cannot fully explain the increase [56]. Identifying additional risk factors, and their synergistic effects, may inform efforts to risk-stratify screening; however, researchers have made little progress in understanding risk factors that may explain young-onset CRC in persons with no family history.
Finally, it is important to recognize that the large, relative increases in young-onset CRC corresponded to an absolute increase of only a few additional cases per 100,000 persons [57]. Rates among younger adults are still low compared with older populations. For example, at age 45–49 years, incidence increased by 36% from 1992–1996 to 2011–2015, but the absolute difference in rates over the same time period is a modest 8.2 cases per 100,000. Considering the absolute risk of CRC in younger adults is important because when the prevalence of disease is very low, even the best screening test will not be an effective public health program [58]. Using a screening test in a population with lower disease prevalence decreases the positive predictive value—and increases the number of false positives. Although there is no consensus on what constitutes “very low” disease prevalence, CRC incidence is markedly lower in certain population subgroups (e.g., white, premenopausal women) than in others. Consequently, the balance of screening benefits and harms will shift when screening a population with lower rates of disease.
Prevalence of Colonic Neoplasia in Younger Adults
Screening works best when the natural history of the disease, from latent to symptomatic disease, is adequately understood [59]. This ensures earlier diagnosis and treatment confers a clinical benefit to the patient. For CRC, risk and prevalence of neoplasia across the adenoma-carcinoma sequence [60, 61], including time from initial development of adenoma to preclinical to clinical disease, form the basis of our understanding of natural history. The goal of CRC screening is to intervene upon the life history of CRC by detecting and removing adenomas that may eventually transition to cancer.
A challenge to describing the natural history of young-onset CRC is understanding the prevalence of colonic neoplasia in younger adults. Most estimates derive from autopsy studies performed decades ago [62,63,64]—and few among 40-year olds. Limited evidence suggests the prevalence of large polyps may be similar between adults ages 40–49 [65] and 50–59 years [66] (3.5% vs. 5.3%). A recent cross-sectional study in Korea shows a very small proportion of 20–29-year olds (0.6%) and 30–39-year olds (0.9%) have advanced neoplasia at colonoscopy [67•]. It is not clear whether earlier removal of these lesions impacts important endpoints, like mortality, in younger age groups. An additional challenge is that many colonoscopies performed in younger adults are among those with symptoms or at higher risk of CRC (i.e., due to family history), and the number of lesions identified from these colonoscopies likely does not reflect the true underlying prevalence of neoplasia in this age group. As a result, we know little about whether asymptomatic lesions in younger adults are more or less aggressive or follow a different disease course than those diagnosed in older adults. Information about the expected course and prognosis of young-onset CRC is often extrapolated from the behavior of adenomas and cancers detected in older (asymptomatic) adults.
Benefits and Harms of CRC Screening
Screening Benefits
The most direct way of establishing benefits of a screening test is through a randomized trial demonstrating reduction in mortality, or at the very least, reduction in important morbidity [58]. In the 1990s, three trials [17, 18, 68] demonstrated the effectiveness of gFOBT in reducing CRC mortality. Around the same time, case-control studies [69, 70] of sigmoidoscopy showed reductions in CRC mortality of up to 60%. Trials of once-only sigmoidoscopy [19, 20, 71, 72], published after 2010, supported results from these early case-control studies. Although trials of screening colonoscopy are still underway [44,45,46], support for colonoscopy has evolved from evidence of gFOBT and sigmoidoscopy established in randomized trials, as well as observational studies demonstrating reductions in CRC incidence and mortality [22, 23]. Consensus quickly developed among professional organizations, and in 1996, the US Preventive Services Task Force endorsed for the first time CRC screening with gFOBT and/or sigmoidoscopy, colonoscopy, or double-contrast barium enema in men and women age 50 years or older [73].
In contrast to evidence accumulated in older populations, very few studies have evaluated CRC screening efficacy in younger adults. Most of the landmark trials of gFOBT and/or sigmoidoscopy are limited to adults older than age 50 years. Notably, the Nottingham trial included adults between the ages of 45 and 74 years and found no mortality benefit of biennial gFOBT among those randomized at age < 60 years (RR 0.96, 95% CI 0.85, 1.10) [38]. The trial was not powered to detect differences in outcome by age, but this finding may suggest benefits of screening are most reliably observed in older adults at average risk. Observational studies supporting the effectiveness of screening are also limited to older adults [37]. In clinical practice, most young adults receiving colonoscopy often do so because of symptoms, family history, or reasons other than screening; therefore, it is difficult to determine screening benefits and yield in a younger, asymptomatic population.
To address this lack of data, experts rely on modeling studies. Modeling studies are not new to cancer screening, and in fact, CRC screening may be particularly well suited for models because of data inputs readily available from randomized trials of gFOBT and sigmoidoscopy and observational studies. For example, in the absence of head-to-head comparisons of different screening tests (e.g., colonoscopy vs. FIT), simulation models have shown several screening strategies reduce CRC mortality by a similar magnitude [3, 74••]. Healthcare organizations have long used modeling studies to make clinical recommendations and reimbursement decisions, including the US Preventive Services Task Force, Centers for Medicare and Medicaid Services, and World Health Organization.
Of course, simulation models perform only as well as the data inputs are accurate. Models often incorporate assumptions not based on any real evidence (e.g., 100% screening adherence, natural history derived from decades’ old autopsy studies), and these assumptions may lead to unrealistic recommendations. Examining the choice and consequences of model-recommended strategies vs. empirical evidence is left to the discretion of the guideline-making organization. Two of the three simulation models used by the US Preventive Services Task Force recommended screening initiation at age 45 years [74••], but given the limited empirical data to support screening 45–49 year olds, the Task Force presented screening strategies with the age to initiate screening of 50 or 55 years. Using the same three simulation models, albeit with slightly different data inputs, the ACS recommended screening initiation at age 45 years. These differing recommendations underscore the importance of exercising caution when allowing modeling studies to guide health policy decisions affecting millions, particularly in the absence of empirical evidence.
Screening Harms
Harms of a screening test can affect multiple domains, from medical complications to anxiety over abnormal results to a cascade of follow-up tests and treatment. Because colonoscopy is the most common (and invasive) screening test, harms of CRC screening are typically measured as the number of lifetime colonoscopies and any resulting complications, such as colonic perforation or major bleeding. Guidelines recommending screening initiation at age 50 years estimate about 4100 lifetime colonoscopies per 1000 persons screened [74••]. Lowering the screening age to 45 years requires an additional 1400 colonoscopies for a total of 5600 lifetime colonoscopies per 1000 screened [29••]. These estimates assume screening occurs predominantly by colonoscopy, with few or no stool-based tests or CT colonographies. Lifetime colonoscopies can be an ambiguous screening harm, particularly to patients, and the impact on clinical practice of additional colonoscopies required by new guidelines is not yet clear.
Other studies of CRC screening harms describe incidence of perforation and bleeding, mostly derived from adverse events reported in trials [75] or large, population-based cohort studies [42, 76]. Risk of screening harms generally increases with age [4••], but few studies describe these harms specifically in younger adults. Among those that do, risk of perforation and bleeding is slightly lower or about the same as in older populations (risk of perforation: 6 per 10,000 colonoscopies among ages 18–49 years [39], risk of bleeding: 2 per 1000 colonoscopies [42]; see Table 2).
Another dimension of screening harms is cost. The US Preventive Services Task Force and other guideline panels make the deliberate decision to exclude cost from assessments of screening benefits and harms, in part to avoid the appearance that screening guidelines limit healthcare based on cost [77]. However, cost is still an important consideration for patients, payers, and healthcare systems, particularly in the USA because insurance coverage widely varies. Although there is no formal cost-effectiveness analysis of initiating screening at age 45 years, for illustration, we can assume screening with a mix of colonoscopy and stool-based tests costs $250 per person and reduces CRC mortality by 50% [33•]. The direct cost to prevent 13,600 CRC deaths among 45–75-year olds would be $2.0 million per death averted, compared with about $1.8 million per death averted when screening ages 50–75 years. There may also be indirect costs to the patient (e.g., time off work, lost productivity) and healthcare system (e.g., shifting diagnostic colonoscopies to screening colonoscopies [31•]). Indirect costs may be especially important to adults in their 40s—an age group arguably in the most productive years of their life [27••].
Conclusion
The goal of any population-wide screening program should be to match risk of disease with the benefits and harms of screening. It is tempting to believe that, because more young adults are diagnosed with CRC, screening must have an equally as large impact. Increases in young-onset CRC are real and important, but increases in incidence alone do not provide any evidence supporting the efficacy of screening—or the potential harms of screening that need to be offset by benefits to be useful. Uncertainty surrounding the efficacy of CRC screening in younger adults, combined with their much lower incidence of CRC, suggests the benefits of screening all 22 million 45–49-year olds may be small.
Moving Toward Precision CRC Screening
Rather than debate the age to initiate CRC screening, we could instead learn from a discussion of how to better define subgroups at increased risk—a concept now referred to as precision cancer screening [78••]. Precision screening uses a combination of genetic factors, environmental and lifestyle exposures, and prior screening to determine the expected benefit of screening for any individual person. Indeed, some organizations have already recommended earlier CRC screening for higher-risk subgroups: African Americans and Alaska Natives, those with family history of CRC or advanced adenomas, and those with inflammatory conditions such as Crohn’s disease or ulcerative colitis (Table 1). In our current clinical practice, we can and should do a better job of identifying those at higher risk, particularly because familial risk (genetic syndrome, family history of either CRC or advanced adenomas) accounts for nearly half of cases diagnosed in younger adults. Meanwhile, research efforts must focus on better understanding the biology of young-onset CRC and associated risk factors, which will facilitate and expand risk-stratified screening in the future.
We should also be mindful of the challenges of precision cancer screening. There are clearly some 45-year olds who will benefit from earlier CRC screening, but identifying those at higher risk (beyond the subgroups listed above) from a pool of 22 million is difficult. Implementing precision screening must take into account a number of factors: endoscopic capacity, management of additional, screen-detected cancers, availability of genetic and environmental information to calculate risk, communication strategies for patients and providers, and the potential impact on disparities. Considering these challenges while the evidence base still accumulates may allow for rapid uptake if precision strategies prove beneficial [78••].
Better risk assessment—and modified screening regimens based on risk—may improve the ratio of benefit to harm over conventional age-based strategies. As genetic information becomes increasingly available, efforts are underway to develop and validate models of CRC risk based on lifestyle, environmental, and genetic risk factors [79•], with hopes of identifying the optimal age to begin screening. For example, a recent study showed risk calculation models that included genetic and environmental factors that have greater accuracy than family history and age alone [79•]. Our goal should be to translate these scientific findings into actionable, clinical information that informs precision cancer screening. Small improvements in risk calculation models can translate into large improvements in risk stratification and recommendations for the age to initiate CRC screening.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Winawer SJ, Miller DG, Schottenfeld D, Leidner SD, Sherlock P, Befler B, et al. Feasibility of fecal occult-blood testing for detection of colorectal neoplasia: debits and credits. Cancer. 1977;40(5 Suppl):2616–9.
National Cancer Institute. Working guidelines for early cancer detection: rationale and supporting evidence to decrease mortality. Bethesda, MD: National Cancer Institute Bethesda; 1987.
Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–69.
•• Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O'Connor E, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576–94. https://doi.org/10.1001/jama.2016.3332 Systematic review commissioned by the U.S. Preventive Services Task Force to update CRC screening recommendations in 2016; addresses effectiveness of screening, test performance, and adverse effects of different screening tests.
Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clin Gastroenterol Hepatol. 2017;15(6):903–9.e6. https://doi.org/10.1016/j.cgh.2016.08.037.
Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31(1):52–61. https://doi.org/10.1016/j.amepre.2006.03.012.
Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med. 2006;31(1):80–9. https://doi.org/10.1016/j.amepre.2006.03.009.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2017 Sub (1973–2015) <Katrina/Rita Population Adjustment> − Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
• Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–75 Summary of 2016 U.S. Preventive Services Task Force recommendation for colorectal cancer screening.
Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on colorectal cancer. Am J Gastroenterol. 2017;112(7):1016–30.
Leddin D, Lieberman DA, Tse F, Barkun AN, Abou-Setta AM, Marshall JK, et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: The Canadian Association of Gastroenterology Banff Consensus. Gastroenterology. 2018;155(5):1325–47.e3. https://doi.org/10.1053/j.gastro.2018.08.017.
Provenzale D, Gupta S, Ahnen DJ, Markowitz AJ, Chung DC, Mayer RJ, et al. NCCN guidelines insights: colorectal cancer screening, version 1.2018. J Natl Compr Cancer Netw. 2018;16(8):939–49.
Gupta S, Provenzale D, Regenbogen SE, Hampel H, Slavin TP Jr, Hall MJ, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 3.2017. J Natl Compr Cancer Netw. 2017;15(12):1465–75. https://doi.org/10.6004/jnccn.2017.0176.
Qaseem A, Denberg TD, Hopkins RH, Humphrey LL, Levine J, Sweet DE, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156(5):378–86.
Canaditaion Task Force on Preventive Health. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188(5):340–8.
•• Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68(4):250–81. Summary of new guidelines by the American Cancer Society recommending average risk colorectal cancer screening begin at age 45 years.
Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–7. https://doi.org/10.1016/s0140-6736(96)03386-7.
Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–71. https://doi.org/10.1016/s0140-6736(96)03430-7.
Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. https://doi.org/10.1016/s0140-6736(10)60551-x.
Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian randomized controlled trial--SCORE. J Natl Cancer Inst. 2011;103(17):1310–22. https://doi.org/10.1093/jnci/djr284.
Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9(12):e1001352. https://doi.org/10.1371/journal.pmed.1001352.
Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8.
Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–105. https://doi.org/10.1056/NEJMoa1301969.
Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, et al. Advances in fecal occult blood tests: the FIT revolution. Dig Dis Sci. 2015;60(3):609–22. https://doi.org/10.1007/s10620-014-3445-3.
Ransohoff DF, Sox HC. Clinical practice guidelines for colorectal cancer screening: new recommendations and new challenges. JAMA. 2016;315(23):2529–31. https://doi.org/10.1001/jama.2016.7990.
Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97–118.
•• Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). https://doi.org/10.1093/jnci/djw322 Landmark publication describing increasing rates of colorectal cancer incidence among younger adults.
Meester RG, Peterse EF, Knudsen AB, de Weerdt AC, Chen JC, Lietz AP, et al. Optimizing colorectal cancer screening by race and sex: microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124:2974–85.
•• Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018;124(14):2964–73. Results of microsimulation modeling studies used to inform 2018 American Cancer Society recommendations for colorectal cancer screening in average-risk adults.
• Corley DA, Peek RM. When should guidelines change? a clarion call for evidence regarding the benefits and risks of screening for colorectal cancer at earlier ages. Gastroenterology. 2018;155(4):947–9 Editorial calling for more evidence on the benefits and harms of colorectal cancer screening for 45–49 year olds.
• Liang PS, Allison J, Ladabaum U, Martinez ME, Murphy CC, Schoen RE, et al. Potential intended and unintended consequences of recommending initiation of colorectal cancer screening at age 45 years. Gastroenterology. 2018;155(4):950–4. https://doi.org/10.1053/j.gastro.2018.08.019 Commentary summarizing the pros and cons of lowering the colorectal cancer screening age to 45 years.
• Anderson JC, Samadder JN. To screen or not to screen adults 45–49 years of age: that is the question. Am J Gastroenterol 2018;113(12):1750–3. Summary of two opposing view points in support of or oppostion to American Cancer Society guidelines for colorectal cancer screening.
• Bretthauer M, Kalager M, Weinberg DS. From colorectal cancer screening guidelines to headlines: beware! Ann Intern Med. 2018;169:405–6 Commentary cautioning against adopting new guidelines to lower the screening age to 45 years.
• Imperiale TF, Kahi CJ, Rex DK. Lowering the starting age for colorectal cancer screening to 45 years: who will come...and should they? Clin Gastroenterol Hepatol. 2018;16(10):1541–4. https://doi.org/10.1016/j.cgh.2018.08.023 Commentary addressing key questions to consider when making recommendations for average-risk screening.
Neugut AI, Jacobson JS, Rella VA. Prevalence and incidence of colorectal adenomas and cancer in asymptomatic persons. Gastrointest Endosc Clin N Am. 1997;7(3):387–99.
Imperiale TF, Abhyankar PR, Stump TE, Emmett TW. Prevalence of advanced, precancerous colorectal neoplasms in black and white populations: a systematic review and meta-analysis. Gastroenterology. 2018;155(6):1776–86.e1. https://doi.org/10.1053/j.gastro.2018.08.020.
Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155(5):1383–91.e5. https://doi.org/10.1053/j.gastro.2018.07.017.
Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61(7):1036–40. https://doi.org/10.1136/gutjnl-2011-300774.
Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69(3):654–64.
Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135(6):1899–906, 906.e1. https://doi.org/10.1053/j.gastro.2008.08.058.
Brenner H, Altenhofen L, Stock C, Hoffmeister M. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clin Gastroenterol Hepatol. 2015;13(4):717–23.
Rutter CM, Johnson E, Miglioretti DL, Mandelson MT, Inadomi J, Buist DS. Adverse events after screening and follow-up colonoscopy. Cancer Causes Control. 2012;23(2):289–96. https://doi.org/10.1007/s10552-011-9878-5.
Murphy CC, Lund JL, Sandler RS. Young-onset colorectal cancer: earlier diagnoses or increasing disease burden? Gastroenterology. 2017;152(8):1809–12.e3. https://doi.org/10.1053/j.gastro.2017.04.030.
Kaminski MF, Bretthauer M, Zauber AG, Kuipers EJ, Adami H-O, van Ballegooijen M, et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012;44(7):695.
Castells A, Quintero E. Programmatic screening for colorectal cancer: the COLONPREV study. Dig Dis Sci. 2015;60(3):672–80.
Dominitz JA, Robertson DJ, Ahnen DJ, Allison JE, Antonelli M, Boardman KD, et al. Colonoscopy vs. fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM): rationale for study design. Am J Gastroenterol. 2017;112(11):1736.
Rex DK, Khan AM, Shah P, Newton J, Cummings OW. Screening colonoscopy in asymptomatic average-risk African Americans. Gastrointest Endosc. 2000;51(5):524–7.
Chauvenet M, Cottet V, Lepage C, Jooste V, Faivre J, Bouvier AM. Trends in colorectal cancer incidence: a period and birth-cohort analysis in a well-defined French population. BMC Cancer. 2011;11:282. https://doi.org/10.1186/1471-2407-11-282.
Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969-2010. Cancer Epidemiol. 2016;42:90–100. https://doi.org/10.1016/j.canep.2016.03.009.
Haggar FA, Preen DB, Pereira G, Holman CD, Einarsdottir K. Cancer incidence and mortality trends in Australian adolescents and young adults, 1982-2007. BMC Cancer. 2012;12:151. https://doi.org/10.1186/1471-2407-12-151.
Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17–22. https://doi.org/10.1001/jamasurg.2014.1756.
•• Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3(4):464–71. https://doi.org/10.1001/jamaoncol.2016.5194 Population-based cohort study in Ohio demonstrating the frequency and wide spectrum of germline muations among young adults diagnosed with colorectal cancer.
•• Stoffel EM, Koeppe E, Everett J, Ulintz P, Kiel M, Osborne J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology. 2018;154(4):897–905.e1. https://doi.org/10.1053/j.gastro.2017.11.004 Large, single center study demonstrating 1 in 5 persons diagnosed with colorectal cancer at age younger than 50 years carries a germline mutation associated with their cancer.
Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology. 2018;155:1716–1719.e4. https://doi.org/10.1053/j.gastro.2018.07.045.
Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5(1):37–44.
Imperiale TF, Kahi CJ, Stuart JS, Qi R, Born LJ, Glowinski EA, et al. Risk factors for advanced sporadic colorectal neoplasia in persons younger than age 50. Cancer Detect Prev. 2008;32(1):33–8. https://doi.org/10.1016/j.cdp.2008.01.003.
Murphy CC, Sanoff HK, Stitzenberg KB, Baron JA, Sandler RS, Yang YC, et al. RE: Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109(8). https://doi.org/10.1093/jnci/djx104.
Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Semin Oncol. 2010;37(3):202–15. https://doi.org/10.1053/j.seminoncol.2010.05.006.
Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–32.
Morson B. The polyp-cancer sequence in the large bowel. Thousand Oaks: SAGE Publications; 1974.
Arminski TC, McLean DW. Incidence and distribution of adenomatous polyps of the colon and rectom based on 1,000 autopsy examinations. Dis Colon Rectum. 1964;7:249–61.
Blatt LJ. Polyps of the colon and rectum. Dis Colon Rectum. 1961;4(4):277–82.
Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157(2):223–6.
Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346(23):1781–5. https://doi.org/10.1056/nejm200206063462304.
Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300(12):1417–22. https://doi.org/10.1001/jama.300.12.1417.
• Jung YS, Ryu S, Chang Y, Yun KE, Park JH, Kim HJ, et al. Risk factors for colorectal neoplasia in persons aged 30 to 39 years and 40 to 49 years. Gastrointest Endosc. 2015;81(3):637-45.e7. https://doi.org/10.1016/j.gie.2014.09.031 Large study of asymptomatic patients undergoing routine colorectal cancer screening showing low prevalence of colonic neoplasia in young adults.
Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer control study. N Engl J Med. 1993;328(19):1365–71. https://doi.org/10.1056/nejm199305133281901.
Selby JV, Friedman GD, Quesenberry CP Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653–7. https://doi.org/10.1056/nejm199203053261001.
Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst. 1992;84(20):1572–5.
Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312(6):606–15.
Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345–57.
U.S. Preventive Services Task Force. Guide to clinical preventive services. Alexandria, VA: International Medical Publishing; 1996.
•• Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016. https://doi.org/10.1001/jama.2016.6828 Results of microsimulation modeling studies used to inform 2016 U.S. Preventive Services Task Force recommendations for colorectal cancer screening in average-risk adults.
Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366(8):697–706. https://doi.org/10.1056/NEJMoa1108895.
Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–6.
U.S. Preventive Services Task Force. USPSTF and Cost Considerations. 2017. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-and-cost-considerations. Accessed December 2018.
•• Marcus PM, Pashayan N, Church TR, Doria-Rose VP, Gould MK, Hubbard RA, et al. Population-based precision cancer screening: a symposium on evidence, epidemiology, and next steps. Cancer Epidemiol Biomarkers Prev. 2016;25(11):1449–55. https://doi.org/10.1158/1055-9965.Epi-16-0555 Review of National Cancer Institue-sponosred symposium on precision cancer screening, including a discussion of available evidence and challenges for implementation.
• Jeon J, Du M, Schoen RE, Hoffmeister M, Newcomb PA, Berndt SI, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology. 2018;154(8):2152-64.e19. https://doi.org/10.1053/j.gastro.2018.02.021 Risk calculation models using a combination of lifestyle and environmental factors and genetic variants determin risk of CRC and optimal age to begin screening.
Funding
National Center for Advancing Translational Sciences (KL2TR001103) and National Cancer Institute (P30CA142543) at the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Caitlin Murphy declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on GI Oncology
Rights and permissions
About this article
Cite this article
Murphy, C.C. Colorectal Cancer in the Young: Does Screening Make Sense?. Curr Gastroenterol Rep 21, 28 (2019). https://doi.org/10.1007/s11894-019-0695-4
Published:
DOI: https://doi.org/10.1007/s11894-019-0695-4