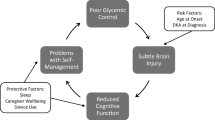
Abstract
The impact of diabetes on the developing brain is well-accepted. Effects on neurocognitive functioning are moderate but have larger functional implications, especially when considered through a developmental lens. Pathophysiological factors such as severe hypoglycemia and chronic hyperglycemia can alter developmental trajectories in early childhood and perhaps at later periods. In this paper, we selectively review neurocognitive outcomes in pediatric diabetes (largely type 1), integrating recent research from developmental neuroscience and neuroimaging. We examine the effects of diabetes at different stages and place findings within a neurodevelopmental diathesis/stress framework. Early-onset diabetes is associated with specific effects on memory and more global cognitive late-effects, but less is known about cognitive outcomes of diabetes in later childhood and in adolescence, a time of increased neurobehavioral vulnerability that has received relatively limited empirical attention. Studies are also needed to better elucidate risk and protective factors that may moderate neurodevelopmental outcomes in youth with diabetes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rice D, Barone Jr S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–33.
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48.
Pechtel P, Pizzagalli D. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2010;214:1–3.
Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol. 2004;10:36–52.
Ryan C. Why is cognitive dysfunction associated with the development of diabetes early in life? The diathesis hypothesis. Pediatr Diabetes. 2006;7:289–97.
Ryan C. Searching for the origin of brain dysfunction in diabetic children: going back to the beginning. Pediatr Diabetes. 2008;9:527–30.
Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184–90.
Armstrong FD. Neurodevelopment and chronic illness: mechanisms of disease and treatment. Ment Retard Dev Disabil Res Rev. 2006;12:168–73.
Mulhern RK, Butler RW. Neuropsychological late effects. In: Brown R, editor. Comprehensive handbook of childhood cancer and sickle cell disease: a biopsychosocial approach. New York: Oxford University Press; 2006.
Turkel S, Pao M. Late consequences of chronic pediatric illness. Psychiatr Clin N Am. 2007;30(4):819–35.
Beauchamp MH, Ditchfield M, Maller JJ, et al. Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int J Dev Neurosci. 2011;29:137–43.
Taylor HG. Children with very low birth weight or very preterm birth. In: Yeates KO, Ris MD, Taylor HG, et al., editors. Pediatric neuropsychology: research, theory, and practice. 2nd ed. New York: Guilford; 2010.
Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr 2007;151(1):73–78.
Vlassara H, Brownlee M, Cerami A. Excessive nonenzymatic glycosylation of peripheral and central nervous system myelin components in diabetic rats. Diabetes. 1983;32:670–4.
Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–35.
Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care. 2008;31:1892–7.
Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes—a meta-analysis. J Ped Psychol. 2009;34:271–82.
Northam EA, Anderson PJ, Werther GA, Warne GL, Adler RG, Andrewes D. Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care. 1998;21:379–84.
Northam E, Anderson P, Jacobs R, Hughes M, Warne G, Werther G. Neuropsychological profiles in children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541–6.
Zuckerman M. Vulnerability to psychopathology: a biosocial model. Washington, DC: American Psychological Association; 1999.
Dabelea D, Rewers A, Stafford JM, Standiford DA, Lawrence JM, Saydah S, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth Study. Pediatrics. 2014;133:e938–45. doi:10.1542/peds.2013-2795.
Glaser NS, Wooton-Gorges S, Buonocore M, Marcin JP, Rewers A, Strain J, et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diabetes. 2006;7:75–80.
Perantie DC, Lim A, Wu J, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87–95.
Ghetti S, Lee JK, Sims C, DeMaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156:109–14. A small but well-designed study showing the effect of DKA on memory in children with early-onset disease.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. The most updated version of a highly influential model of the primary mechanisms for hyperglycemia-mediated cellular damage in diabetes and their role in diabetic complications.
Antenor-Dorsey JA, Meyer E, Rutlin J, Perantie DC, White NH, et al. White-matter microstructural integrity in youth with type 1 diabetes. Diabetes. 2013;62:581–9.
Aye T, Reiss AL, Kesler S, et al. The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes Care. 2011;34:1458–62.
Aye T, Barnea-Goraly N, Ambler C, et al. White-matter structural differences in young children with type 1 diabetes: a diffusion tensor imaging study. Diabetes Care. 2012;35:2167–73.
Marzelli MJ, Mazaika PK, Barnea-Goraly N, Hershey T, Tsalikian E, Tamborlane W, et al. Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes. 2014;63:343–53. The most recent neuroimaging findings from a large group of young children with early-onset diabetes, demonstrating a relation between CNS changes and cognitive outcomes.
Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, et al. Alterations in white-matter structure in young children with type 1 diabetes. Diabetes Care. 2014;37:332–40. The largest DTI study of a pediatric T1D cohort to date, demonstrating the effects of dysglycemia on white matter structure and integrity.
Schwartz DD, Axelrad ME, Cline VD, Anderson BJ. Neurocognitive functioning in children and adolescents at the time of type 1 diabetes diagnosis: associations with glycemic control one year after diagnosis. Diabetes Care 2014, doi:10.2337/dc14-0103.
Cato M, Mauras N, Ambrosino J, Bondurant A, Conrad AL, Kollman C, et al. Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Soc. 2014;20:238–47.
Peila R, Rodriguez BL, Launer LJ. Type 2 Diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes. 2002;51:1256–62.
Ferguson SC, Deary IJ, Evans JC, Ellard S, Hattersley AT, Frier BM. Apolipoprotein-E influences aspects of intellectual ability in type 1 diabetes. Diabetes. 2003;52:145–8.
Jacobson AM, Ryan CM, Cleary PA, Diabetes Control and Complications Trial/EDIC Research Group, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia. 2011;54:245–55. An 18-year follow-up of the DCCT cohort examining potential moderators of cognitive outcomes including biomedical and genetic factors.
Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61(3):154–66.
Krull KR, Bhojwani D, Conklin HM, Pei D, Cheng C, Reddick WE, et al. Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2182–8.
Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113(4–5):234–58.
Hovnik T, Dolžan V, Bratina NU, Podkrajšek KT, Battelino T. Genetic polymorphisms in genes encoding antioxidant enzymes are associated with diabetic retinopathy in type 1 diabetes. Diabetes Care. 2009;32(12):2258–62.
McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–9.
Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, Paul RH. Impact of early versus late childhood early stress on brain morphometrics. Brain Imaging Behav. 2013;7:196–203.
Ack M, Miller I, Weil WB. Intelligence of children with diabetes mellitus. Pediatrics. 1961;28:764–70.
Vannucci RC, Vannucci SJ. Glucose metabolism in the developing brain. Semin Perinatol. 2000;24:107–15.
Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioral plasticity after early brain insult. Brain. 2011;134:2197–221.
Lin A, Northam EA, Rankins D, Werther GA, Cameron FJ. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatr Diabetes. 2010;11:235–43. The most recent neurocognitive outcome data from a longitudinal study of a well-defined cohort of children with T1D followed since disease onset.
Northam EA, Rankins D, Lin A, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445–50.
Ho MS, Weller NJ, Ives FJ, Carne CL, Murray K, Vanden Driesen RI, et al. Prevalence of structural central nervous system abnormalities in early-onset type 1 diabetes mellitus. J Pediatr. 2008;153:385–90.
Languren G, Montiel T, Julio-Amilpas A, Massieu L. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int. 2013;63:331–43.
Blasetti A, Chiuri RM, Tocco AM, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol. 2011;26:1383–91. The results of this meta-analysis suggest that recurrent severe hypoglycemia has a specific effect on learning and memory with milder effects on other areas of cognition.
Bjørgaas MR. Cerebral effects of severe hypoglycaemia in young people with type 1 diabetes. Pediatr Diabetes. 2012;13:100–7.
Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes. 2010;59:236–41.
Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–44.
Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–8.
Patiño-Fernández AM, Delamater AM, Applegate EB, et al. Neurocognitive functioning in preschool-age children with type 1 diabetes mellitus. Pediatr Diabetes. 2010;11:424–30.
Crone EA, Ridderinkhof RK. The developing brain: from theory to neuroimaging and back. Dev Cogn Neurosci. 2011;1:101–9. A conceptual review of brain development that links neuroscience approaches to more traditional views of cognitive development.
Conklin H, Luciana M, Hooper C, Yarger R. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev Neuropsychol. 2007;31:103–28.
Ostby Y, Tamnes CK, Fjell AM, Walhovd KB. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49:3854–62.
Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LM. Depressive symptoms in children and adolescents with type 1 diabetes: association with diabetes-specific characteristics. Diabetes Care. 2006;29:1389–91.
Anderson BJ. Family conflict and diabetes management in youth: clinical lessons from child development and diabetes research. Diabetes Spec. 2004;17:22–6.
Anderson BJ, Schwartz DD. Psychosocial and family issues in children with type 1 diabetes. In, Umpierrez, G. Therapy for diabetes mellitus and related disorders, 6th Edition. American Diabetes Association (2014). This book chapter reviews psychosocial issues faced by children and youth with diabetes and their families from a developmental perspective.
Steinberg L. Risk-taking in adolescence: new perspectives from brain and behavioral science. Curr Dir Psychol Sci. 2007;16:55–9.
Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Rewers A et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287:2511–8.
Colver A, Longwell S. New understanding of adolescent brain development: relevance to transitional healthcare for young people with long term conditions. Arch Dis Child. 2013;98:902–7.
Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:1028–32.
Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. A brief up-to-date review of recent neuroimaging findings of relevance to adolescent brain development.
Buckner R, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38.
Sisk C, Zehr J. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–74.
Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–91.
Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32:7917–25.
Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white-matter integrity and cognitive function. Child Dev. 2013. doi:10.1111/cdev.12069.
van Duinkerken E, Schoonheim MM, Ijzerman RG, et al. Diffusion tensor imaging in type 1 diabetes: decreased white-matter integrity relates to cognitive functions. Diabetologia. 2012;55:1218–20.
Kaufmann L, Pixner S, Starke M, et al. Neurocognition and brain structure in pediatric patients with type 1 diabetes. J Pediatr Neuroradiol 2011: Neurocognition and brain structure in pediatric patients with type 1 diabetes;
Perantie DC, Wu J, Koller JM, et al. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. 2007;30:2331–7.
Perantie DC, Koller JM, Weaver PM, et al. Prospectively determined impact of type 1 diabetes on brain volume during development. Diabetes. 2011;60:3006–14. The results of this 2-year prospective neuroimaging study revealed within-diabetes group differences in whole brain gray matter associated with hyperglycemia and decreased occipital/parietal white matter associated with hypoglycemia. No differences were found between children with and without T1D.
Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14:541–53.
Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17.
Fox MA, Chen RS, Holmes CS. Gender differences in memory and learning in children with insulin-dependent diabetes mellitus (IDDM) over a 4-year follow-up interval. J Pediatr Psychol. 2003;28:569–78.
Musen G, Jacobson AM, Ryan CM, Clearly PA, Waberski BH, Weinger K, et al. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care. 2008;31:1933–8.
Jacobson AM et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–52.
McNally K, Rohan J, Pendley JS, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33(6):1159–62.
Miller MM, Rohan JM, Delamater A, Shroff-Pendley J, Dolan LM, et al. Changes in executive functioning and self-management in adolescents with type 1 diabetes: a growth curve analysis. J Pediatr Psychol. 2013;38:18–29.
Ohmann S, Popow C, Rami B, et al. Cognitive functions and glycaemic control in children and adolescents with type 1 diabetes. J Psych Med. 2010;40(1):95–103.
Asvold BO, Sand T, Hestad K, Bjorgaas MR. Cognitive function in type 1 diabetic adults with early exposure to severe hypoglycemia: a 16-year follow-up study. Diabetes Care. 2010;33:1945–7.
Wrighten SA, Piroli GG, et al. A look inside the diabetic brain: contributors to diabetes-induced brain aging. Biochim Biophys Acta. 2009;1792(5):444–53.
Bruehl H, Sweat V, Tirsi A, Shah B, Convit A. Obese adolescents with type 2 diabetes mellitus have hippocampal and frontal lobe volume reductions. Neurosci Med. 2011;2:34–42. Along with the companion paper by Yau et al. below, the only examination of the effects of T2D on adolescent brain development.
Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53:2298–306.
Brands AMA, Kessels RPC, Hoogma RP, et al. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes. 2006;55:1800–6.
Schütt M, Fach EM, Seufert J, et al. For the DPV Initiative and the German BMBF Competence Network Diabetes Mellitus. Multiple complications and frequent severe hypoglycaemia in ‘elderly’ and ‘old’ patients with type 1 diabetes. Diabet Med. 2012;29:e176–9.
Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2005;25:1–14.
Duke DC, Harris MA. Executive function, adherence, and glycemic control in adolescents with type 1 diabetes: a literature review. Curr Diab Rep 2014.
Compliance with Ethics Guidelines
Conflict of Interest
David D. Schwartz, Rachel Wasserman, Priscilla W. Powell, and Marni E. Axelrad declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Psychosocial Aspects
Rights and permissions
About this article
Cite this article
Schwartz, D.D., Wasserman, R., Powell, P.W. et al. Neurocognitive Outcomes in Pediatric Diabetes: a Developmental Perspective. Curr Diab Rep 14, 533 (2014). https://doi.org/10.1007/s11892-014-0533-x
Published:
DOI: https://doi.org/10.1007/s11892-014-0533-x