Abstract
Common intestinal infections caused by human enteroviruses (HEVs) are considered major environmental factors predisposing to type 1 diabetes (T1D). In spite of the active research of the field, the HEV-induced pathogenetic processes are poorly understood. Recently, after the first documented report on HEV infections in the pancreatic islets of deceased T1D patients, several groups became interested in the issue and studied valuable human material, the autopsy pancreases of diabetic and/or autoantibody-positive patients for HEV infections. In this review, the data on HEV infections in human pancreatic islets are discussed with special reference to the methods used. Likewise, mechanisms that could increase viral access to the pancreas are reviewed and discussed.
Similar content being viewed by others
Introduction
Type 1 diabetes (TID) is a chronic disease characterized by a progressive and selective destruction of the pancreatic β cells in the islets of Langerhans. The underlying pathogenic processes finally resulting in the inability to control blood glucose due to the lack of insulin synthesis already start years before clinical symptoms become manifest and is characterized by the appearance of islet cell–specific antibodies in the circulation. The incidence of T1D is increasing worldwide. Although genetic predisposition is a risk factor for the acquisition of the T1D, it cannot explain the rapidly increasing incidence and, thus, during the past decades, the role of environmental factors has become clearly evident. Various agents that gain entry into the body through the intestines—including nutrients, microbial toxins, and different microbes—have been studied in relation to T1D. However, the major role for T1D induction seems to be viruses and especially human enteroviruses (HEV). Several coxsackievirus and echovirus serotypes have been associated with the development of T1D in a number of cross-sectional and prospective studies [1–5]. Furthermore, virus strains with the definite capability to destroy pancreatic β cells are found in all studied HEV serotypes in a wide range of in vitro and in vivo studies in islets cell cultures and mice [1, 2, 4, 6]. In human pancreas, HEVs reveal strong affinity to islets suggesting that direct virus interaction with the islets might be of special importance in the pathogenesis of T1D.
Enteroviruses
HEVs, members of the Picornaviridae family, are small nonenveloped virus particles with icosahedral symmetry of the capsid. The RNA genome is surrounded by a protein capsid made up of 60 copies of viral capsid proteins VP1 to VP4. Traditionally, HEVs were known as poliovirus, coxsackievirus, echovirus, and new enteroviruses, but more recently they have been subdivided into four species—referred to as HEV-A to HEV-D—on the basis of their phylogenetic properties [7]. Already more than 100 HEV serotypes are recognized and the number is still increasing since the new sequence-based typing method [8] is unveiling new serotypes among strains that were previously left as nontypable.
HEV Have Affinity to Pancreatic Islets
The main route of HEV contagion is feco-oral. Despite the fact that primary infection involves the gut, the presence of virus in the intestinal mucosa cells has never been convincingly demonstrated by localization techniques, visualizing HEV RNA and proteins in epithelial cells. However, it is known that the virus gains access to the circulation via the lymphatic system and finally to secondary replication sites in different organs. Acute virus replication in these organs determines the type of symptoms caused by the infection. The pancreas is one of the major secondary target tissues for HEVs, as shown by Yoon et al. [9, 10] a long time ago. However, species-specific differences exist between men and mice regarding virus-specific cell tropism because in the mouse pancreas HEV infects exocrine tissue [11], whereas in the human pancreas the infection is restricted to islets. After the first documented report on HEV infections in the pancreatic islets of T1D patients [12], several research groups (Table 1) became interested in the presence of HEV in pancreatic islets and studied the highly valuable material—autopsy pancreases of diabetic [13••, 14••] or autoantibody-positive patients [15••]—for the presence of HEV. Furthermore, in a study by Dotta et al. [13••], an infectious coxsackievirus B4 was isolated from the islets of T1D patients. In addition to the classic T1D, HEV RNA has been detected in β cells [16••] and HEV proteins in islets [17••] of patients with fulminant T1D, which is defined as a novel nonautoimmune subtype of the disease that is relatively common in Japan.
Detection of Enterovirus RNA and Proteins in Human Tissues
Various studies have suggested a relationship of enterovirus infections with the development of T1D applying (quantitative) reverse transcription-polymerase-chain reaction (RT-PCR) methods or blotting techniques in blood, stool samples, and saliva [1, 3, 4]. However, the disadvantage of PCR assays or Northern and Western blot analyses in the diagnosis and research of enterovirus infections in specific diseases is the fact that free virus in the blood system or virus within immune cells cannot be differentiated from replicating virus within organ-specific cell types. Thus, to investigate pathogenetic-relevant mechanisms of enterovirus–host interactions in the induction of diabetes at the cellular level, there is the need to visualize virus-related molecules (RNA and proteins) directly within the pancreatic tissue. The infection of insulin-producing β cells by enteroviruses can only be proven by in situ techniques, capable of detecting viral RNA by in situ hybridization or proteins applying immunohistochemistry.
Sensitivity and Specificity of HEV Detection Methods
Regarding the sensitivity of HEV RNA detection by radioactive in situ hybridization, it was shown that 20 viral copies can be detected in infected cultured cells within 2 weeks of autoradiographic exposure [18]. A further advantage of the technique is the fact that viral RNA is well conserved in formalin-fixed and paraffin-embedded pancreatic tissues and can be detected for more than 30 years in this tissue with a high sensitivity if primary autolysis in the pancreas can be prevented [12]. By using 35S-labeled RNA probes covering the full genome of enteroviruses (7500 bp), it was proven that enteroviral RNA is present in islets and some duct cells not only in the pancreas of babies who died of acute enterovirus infections but also in patients having T1D [12]. The detection of enteroviral RNA in pancreatic tissue by nonradioactive in situ hybridization is not recommended because of problems with high background activity that make it impossible to clearly identify virus RNA-positive cells within the pancreas, especially when oligonucleotides are used as probes [16••].
In addition, we found a highly reduced sensitivity in RNA detection by nonradioactive in situ hybridization as only acutely but no persistently infected cells with low viral copy numbers were detected by this method. Even acutely cultured green monkey kidney cells infected with different enteroviruses including coxsackieviruses and echoviruses often show only weak signals when they are hybridized by nonradioactively labeled probes, as recently reported [19]. In this paper it is also interesting to see that despite the presence of enteroviral genomes, there are considerable differences of the detection of enteroviral proteins in these acutely infected cells dependent on the type of antibodies used. One of the best working antibodies in cell culture is the enterovirus 5-D8/1 clone, which has been used in many studies before for the detection of enteroviral proteins in different tissues. However, as shown in various laboratories by different detection methods, this antibody reacts not only with virus-specific epitopes but also with unrelated cellular proteins (eg, in the heart [20] and also in the pancreas [21••]), indicating that immunohistochemistry alone, with the currently available antibodies is not suitable to prove an enterovirus infection but must be confirmed by the simultaneous detection of viral RNA in these cells. One explanation for the cross-reaction might be the fact that the highly conserved N-terminal immunodominant region of VP1 reveals sequence similarity with the known diabetes-associated epitopes for different islet cell autoantigens (eg, the heat-shock protein 60/65 and the tyrosine phosphatases IA-2/IAR) [22, 23].
Several groups have searched evidence for the presence of enteroviruses by using the enterovirus 5-D8/1 clone in autopsy pancreases of diabetic and/or autoantibody-positive patients and reports on positive findings have been published (Table 1) [13••–15••, 17••]. In the most recent publication, Richardson et al. [14••] reported that 44 autopsy pancreases of 72 recent-onset T1D patients studied had a positive HEV signal in islets. The result was in accordance with the original findings of other groups [12, 13••], thus providing further support for the role of enteroviruses as candidate triggers of T1D. However, the finding that smooth muscle cells are also positive by this antiserum confirms the problem of nonspecific staining, as these cells have never been proven to be infectable by enteroviruses in vivo by visualizing in parallel the viral RNA in these cells. It is also worthy to note that the pancreas is subject to a quick enzyme-mediated postmortem autolysis and this often leads to degradation processes, decreasing the sensitivity of enterovirus detection and increasing the background staining in immunohistochemistry [24]. This might explain the unusual findings in autopsy pancreas of patients with fulminant T1D in which pyknotic acinar cells and not insulin-producing cells are positively stained with the enterovirus 5-D8/1 antibody [17••].
Diabetogenic Mechanisms of HEV
The presence of HEV in islets of human autopsy pancreases of T1D patients suggests that direct interaction of virus with islets cells might be important in the pathogenesis of T1D. There are different but not mutually nonexclusive mechanisms by which HEV infection in pancreatic islets may accelerate or modulate the development of T1D [1, 2, 4, 5].
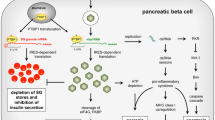
Because HEVs are known as highly cytolytic viruses they may, after getting to the pancreatic islets, destroy insulin-producing β cells by virus-induced cytolysis. In addition, as a consequence of infection, the innate and adaptive immune systems are activated which results in inflammation. Alternatively, it is discussed that HEV-induced β-cell destruction might be based on bystander activation of autoreactive T cells or loss of regulatory T cells.
In addition to a viral infection in the pancreatic islets, local virus-induced production of cytokines and other soluble mediators of the immune system are critical in the pathogenesis of T1D. Most probably, the β-cell fate following viral-induced damage depends on a large number of genes upregulated or downregulated simultaneously with specific changes in cellular metabolites and cytokines. The only present way to unveil these interactions is by using high-throughput methods, such as microarray analysis and metabolomics in which these interfering systems can be examined in parallel. The global profiling of gene expression has already revealed the genes expressed in primary human islets [25] and purified β cells [26] and disclosed a large number of those that are specifically modified by an enterovirus, coxsackievirus B5 [25], or by cytokines (interleukin-1β [IL-1β] and interferon-γ [IFN-γ] in human islets [25] and IL-1β and tumor necrosis factor-α in rat β cells [26]), thus providing novel insights into the molecular mechanisms that might be involved in virus- and cytokine-mediated human β-cell dysfunction and death.
In a recent study in which Schulte et al. [27•] addressed diabetogenic mechanisms of HEV, a new exciting immunologic mechanism was proposed. By using human islets as an experimental model they demonstrated that coxsackievirus-infected islets were efficiently phagocytosed by human monocyte-derived dendritic cells. Moreover, they demonstrated that phagocytosis of coxsackievirus-infected cells induced an antiviral state that protected dendritic cells from subsequent coxsackievirus infection. This antiviral state of protected dendritic cells was dependent on the presence of intracellular viral RNA in the coxsackievirus-infected cells and type 1 IFNs produced by the dendritic cells. As discussed by the authors, these virus-induced effects may alter programming of dendritic cells and, thus, influence the development of regulatory T cells and/or effector T-cell populations [27•]. Although the study by Schulte et al. [27•] identified a mechanism by which HEV-infected islets may affect the local immune balance, it remains to be shown whether this really results in induction of T1D or, alternatively, in protection from autoimmune-mediated destruction in vivo.
Prolonged HEV Infections in T1D Patients
In most individuals, HEV infections are efficiently controlled by an adequate antiviral immune response. Although circulating antibodies will prevent blood-borne spreading of the infection within the body, virus-specific cytotoxic immunity together with mucosal humoral immune response are considered to be important for eliminating the primary virus infection and for protecting the body from future infections. Data from several studies suggest that individuals with high genetic risk for T1D may have impaired antiviral defense mechanisms in virus clearance. It is well documented that HEV RNA can be found in the blood of patients with newly diagnosed T1D but not—or at least with a much lower frequency—in healthy controls [1, 3]. Because the viremia in HEV-infected individuals is usually short and difficult to detect due to low virus loads, these results suggest that patients with T1D may have prolonged enterovirus infection in blood cells, as proposed by Schulte et al. [28•]. They found HEV RNA in peripheral blood mononuclear cells (PBMCs) of T1D patients beyond the stage of acute infection. Furthermore, they showed that HEVs detected at the onset of T1D were not free in plasma but present in PBMCs. These findings raised an interesting question—whether HEVs might use monocytes and/or lymphocytes as viral reservoirs and vehicles for viral dissemination. This hypothesis is underlined by findings in experimental enterovirus infections in which macrophages, B lymphocytes, and T-helper cells were found to harbor coxsackievirus RNA for long periods after infection [11]. Through these mechanisms the viremic phase might be prolonged, thus increasing the possibility of virus to reach secondary target tissues such as the pancreas.
Prolonged detection of viral RNA in leukocytes of T1D patients suggests that these patients may have delayed clearance of HEV. According to the results by Skarsvik et al. [29], children with T1D were found to have a decreased in vitro type 1 immune response against coxsackievirus B4, a fact that may also lead to delayed virus clearance and, at least partially, explain why children with T1D may be more prone to HEV infections and related complications, such as β-cell damage.
The amount of virus in PBMCs might be enhanced by serum antibodies, a mechanism called antibody-dependent enhancement of viral infection. As demonstrated by Hober et al. [30], non-neutralizing antibodies to viral capsid protein VP4 present in human sera are able to enhance both coxsackievirus B4 infection and virus-induced synthesis of IFN-α in human monocytes and/or macrophages. According to their results, the prevalence and the levels of these antibodies were higher among diabetic patients compared with healthy subjects, which suggests a role for these antibodies in the development of T1D [31, 32•]. Because this enhancing mechanism is also dependent on cell surface expression of coxsackie B virus receptor, HCAR (human coxsackie and adenovirus receptor) [30], it is not known whether the corresponding mechanism might work for other enteroviruses with various receptor specificities [33]. However, the possible existence of pathogenic non-neutralizing antibodies has to be taken into account whenever the development of coxsackie and/or enterovirus vaccines is discussed.
The occurrence of a chronic HEV infection was also discussed in a study by Oikarinen et al. [34•], in which immunopositivity to HEV antibody was detected in biopsy samples of small intestines of T1D patients more frequently than in samples from healthy controls. During acute HEV infection the replication of virus in the gut can continue for several weeks up to a couple of months. Viral infections causing vomiting and diarrhea evidently disrupt intestinal barrier integrity. It is possible that infections caused by HEVs are sufficient to increase the intestinal permeability, a phenomenon recently associated with an increased susceptibility to the development of T1D [35•].
As such, an idea of chronically HEV-infected patients is not new because it is well known that sometimes, when patients with hypogammaglobulinemia are infected by oral live poliovirus vaccine, the virus excretion may continue in intestinal mucosa for several years or for the rest of their lives [36•]. Likewise, the patients with hypogammaglobulinemia may encounter the same problems with other nonpolio-enteroviruses; however, it is unclear whether genetic susceptibility to T1D renders these patients more vulnerable to chronic intestinal infections. In the study by Oikarinen et al. [34•], enteroviral antigen-positive intestinal epithelial cells were detected by an immunostaining for capsid protein VP1 and by nonradioactive in situ hybridization using short oligonucleotide probes. As discussed above, both used methods are well known to have problems in terms of unspecific reactions in diverse human tissues including the intestine, thus possibly explaining the discrepancy of RNA and protein detection in the gut of T1D patients in their study. Therefore, these results should be confirmed by demonstrating the presence of infectious virus by virus isolation or by visualizing the presence of virus genomes by in situ hybridization using a radioactive probe in the same cells that are positive for viral protein using consecutive tissue sections.
Diabetogenic Properties of Enteroviruses
Results from the prospective studies—human islet in vitro studies and animal studies—indicate that various HEV serotypes might be able to modulate the development of T1D. Although the strongest evidence is available from coxsackieviruses and echoviruses of HEV-B species, it is not possible to conclude, due to lack of evidence, that other serotypes of HEV-B or other HEV species are less diabetogenic.
HEV infections are among the most common viral infections. Most of the infections are subclinical or mild with common cold-like symptoms, eye infections, skin disease, and so forth. In some cases, however, HEV infection causes a more severe disease including myocarditis, neurologic involvements, acute hemorrhagic conjunctivitis, pleurodynia, and neonatal sepsis-like disease. It is not known what defines the severity and course of an enterovirus infection. Likewise, it is not clear whether the children who are genetically susceptible to T1D will become infected with the regular HEV strains circulating in communities or whether they are more prone to infections caused by specific, more diabetogenic virus variants. Therefore, the major question is what makes an HEV diabetogenic?
Current knowledge suggests that the diabetogenic properties of HEV strains are not defined by the serotype but by other characteristics of virus strains [37]. In several studies the molecular determinants of the virus strains providing increased islet cell tropism, pathogenicity, and/or virulence have been searched for by complete genome sequence analyses. However, the published full-length genome sequences of the virus isolates did not disclose the most critical determinants inducing diabetes [38, 39•, 40•, 41–44]. As such, this is no surprise because a typical feature of HEV evolution is a rapid accumulation of point mutations and frequent recombination. In practice, this means that clinical isolates of HEVs are mixtures of microvariants. Although these variants diverge from each other by only a few nucleotides, they may have differences in tissue tropism and virulence. The quasispecies nature of HEV is considered essential for their capacity to adapt to new environment. As suggested by recent studies by Al-Hello et al. [40•], in murine insulin-producing β cells (MIN-6) in vitro a single amino acid of capsid protein VP1 may be responsible for cell tropism and cytopathology of coxsackievirus B5.
Recombination can take place among genetically closely related virus strains when they coinfect the same host cell. In this process, viruses achieve larger fragments of new genetic material that might be beneficial in their rapid evolution, thus enabling them to adapt to a variety of new environmental conditions. When genetic and phenotypic features of echovirus 11 strain isolated from a diabetic child were studied, the results revealed interesting characteristics typical for virus recombinants. The isolate, initially serotyped as coxsackievirus A9, actually harbors double serotype specificity and shares this property with a previously described subgroup of echovirus 11 strains [39•].
During recent years HEV activity has increased globally [45–48]. Several new variants with potentially increased virulence have been reported and identified as a causative agent of an epidemic [49, 50]. However, no data are available on their risk to induce T1D.
Conclusions
There is firm evidence that on top of a strong genetic component, environmental factors are the decisive elements contributing to the increasing incidence of T1D. Although intestinal HEV infections are considered to be major environmental factors predisposing to T1D, the underlying virus-induced pathogenetic processes have been poorly recognized. The documented presence of HEVs in autopsy pancreases of T1D patients highlights the possibility that direct interaction of virus with pancreatic islets and insulin-producing β cells is important in development of T1D. Given the potential importance of the results obtained from human autopsy tissues with regard to virus-host interactions in T1D, special attention should be devoted to the evaluation of the methods used for HEV detection in patients’ tissues.
When hard evidence on the viral exposure hypothesis in the pathogenesis of T1D can be obtained and critical enterovirus serotypes identified, new possibilities to specific interventions could be found. For example, an enterovirus vaccine might be able to reduce the risk of T1D development in individuals immunized soon after birth. However, to be effective, the vaccine should not only diminish acute enterovirus diseases but also prevent asymptomatic infections. Because most of the enterovirus infections are mild or asymptomatic, a putative enterovirus vaccine should be multivalent and contain the most prevalent serotypes. During weeks-long replication in the human gut enteroviruses are excreted into feces and translocated to the environment and, therefore, environmental surveillance (ie, monitoring sewage samples for enteroviruses) can be used as an important tool in studies on enterovirus prevalence and epidemiology.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hober D, Sauter P: Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol 2010, 6:279–289.
Roivainen M: Enteroviruses: new findings on the role of enteroviruses in type 1 diabetes. Int J Biochem Cell Biol 2006, 38:721–725.
Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H: Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol 2010 Apr 28 [Epub ahead of print].
Richer MJ, Horwitz MS: Coxsackievirus infection as an environmental factor in the etiology of type 1 diabetes. Autoimmun Rev 2009, 8:611–615.
Richer MJ, Horwitz MS: The innate immune response: an important partner in shaping coxsackievirus-mediated autoimmunity. J Innate Immun 2009, 1:421–434.
Filippi CM, von Herrath MG: 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: viruses, autoimmunity and immunoregulation. Clin Exp Immunol 2010, 160:113–119.
King AM, Brown F, Christian P, et al.: Picornaviridae. In Virus Taxonomy: Seventh Report of the International Committee on Taxonomy of Viruses. Edited by Van Regenmortel MH, Fauquet, CM, Bishop DH, et al. San Diego, CA: Academic Press; 2000:657–678.
Oberste MS, Maher K, Kilpatrick DR, et al.: Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol 1999, 37:1288–1293.
Yoon JW, Austin M, Onodera T, Notkins AL: Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med 1979, 300:1173–1179.
Yoon JW, Notkins AL: Virus-induced diabetes in mice. Metabolism 1983, 32:37–40.
Klingel K, Stephan S, Sauter M, et al.: Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. J Virol 1996, 70:8888–8895.
Ylipaasto P, Klingel K, Lindberg AM, et al.: Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004, 47:225–239.
•• Dotta F, Censini S, van Halteren AG, et al.: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007, 104:5115–5120. This paper provides evidence for the presence of enteroviruses in pancreatic islets.
•• Richardson SJ, Willcox A, Bone AJ, et al.: The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009, 52:1143–1151. This paper provides evidence for the presence of enteroviruses in pancreatic islets.
•• Oikarinen M, Tauriainen S, Honkanen T, et al.: Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia 2008, 51:1796–1802. This paper provides evidence for the presence of enteroviruses in pancreatic islets.
•• Shibasaki S, Imagawa A, Tauriainen S, et al.: Expression of toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J 57:211–219. This paper provides evidence for the presence of enteroviruses in pancreatic islets.
•• Tanaka S, Nishida Y, Aida K, et al.: Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes 2009, 58:2285–2291. This paper provides evidence for the presence of enteroviruses in pancreatic islets.
Klingel K: Molecular biologic detection of virus infection in myocarditis and dilated cardiomyopathy. In Myocarditis. Edited by Cooper LT. Totowa, NJ: Humana Press; 2002:295–324.
Oikarinen M, Tauriainen S, Penttila P, et al.: Evaluation of immunohistochemistry and in situ hybridization methods for the detection of enteroviruses using infected cell culture samples. J Clin Virol 47:224–228.
Klingel K, Sauter M. Bock CT, et al.: Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol 2004, 193:101–107.
•• Roivainen M, Klingel K: Role of enteroviruses in the pathogenesis of type 1 diabetes. Diabetologia 2009, 52:995–996. This paper provides evidence for the presence of enteroviruses in pancreatic islets.
Harkonen T, Lankinen H, Davydova B, et al.: Enterovirus infection can induce immune responses that cross-react with beta-cell autoantigen tyrosine phosphatase IA-2/IAR. J Med Virol 2002, 66:340–350.
Harkonen T, Puolakkainen M, Sarvas M, et al.: Picornavirus proteins share antigenic determinants with heat shock proteins 60/65. J Med Virol 2000, 62:383–391.
Bussolati G, Leonardo E: Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J Clin Pathol 2008, 61:1184–1192.
Ylipaasto P, Kutlu B, Rasilainen S, et al.: Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 2005, 48:1510–1522.
Ortis F, Naamane N, Flamez D, et al.: Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes 59:358–374.
• Schulte BM, Kramer M, Ansems M, et al.: Phagocytosis of enterovirus-infected pancreatic beta-cells triggers innate immune responses in human dendritic cells. Diabetes 59:1182–1191. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
• Schulte BM, Bakkers J, Lanke KH, et al.: Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral Immunol 23:99–104. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
Skarsvik S, Puranen J, Honkanen J, et al.: Decreased in vitro type 1 immune response against coxsackie virus B4 in children with type 1 diabetes. Diabetes 2006, 55:996–1003.
Hober D, Chehadeh W, Bouzidi A, Wattre P: Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-alpha synthesis. J Infect Dis 2001, 184:1098–1108.
Chehadeh W, Lobert PE, Sauter P, et al.: Viral protein VP4 is a target of human antibodies enhancing coxsackievirus B4- and B3-induced synthesis of alpha interferon. J Virol 2005, 79:13882–13891.
• Sauter P, Chehadeh W, Lobert PE, et al.: A part of the VP4 capsid protein exhibited by coxsackievirus B4 E2 is the target of antibodies contained in plasma from patients with type 1 diabetes. J Med Virol 2008, 80:866–878. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
Ylipaasto P, Eskelinen M, Salmela K, et al.: Vitronectin receptors, alpha v integrins, are recognized by several non-RGD-containing echoviruses in a continuous laboratory cell line and also in primary human Langerhans’ islets and endothelial cells. J Gen Virol 91:155–165.
• Oikarinen M, Tauriainen S, Honkanen T, et al.: Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol 2008, 151:71–75. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
• Vaarala O: Leaking gut in type 1 diabetes. Curr Opin Gastroenterol 2008, 24:701–706. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
• Minor P: Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine 2009, 27:2649–2652. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
Roivainen M, Ylipaasto P, Savolainen C, et al.: Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 2002, 45:693–702.
Al-Hello H, Davydova B, Smura T, et al.: Phenotypic and genetic changes in coxsackievirus B5 following repeated passage in mouse pancreas in vivo. J Med Virol 2005, 75:566–574.
• Al-Hello H, Paananen A, Eskelinen M, et al.: An enterovirus strain isolated from diabetic child belongs to a genetic subcluster of echovirus 11, but is also neutralised with monotypic antisera to coxsackievirus A9. J Gen Virol 2008, 89:1949–1959. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
• Al-Hello H, Ylipaasto P, Smura T, et al.: Amino acids of Coxsackie B5 virus are critical for infection of the murine insulinoma cell line, MIN-6. J Med Virol 2009, 81:296–304. This paper provides evidence for the mechanisms by which enterovirus infections might be involved in the pathogenesis of T1D.
Kang Y, Chatterjee NK, Nodwell MJ, Yoon JW: Complete nucleotide sequence of a strain of coxsackie B4 virus of human origin that induces diabetes in mice and its comparison with nondiabetogenic coxsackie B4 JBV strain. J Med Virol 1994, 44:353–361.
Paananen A, Ylipaasto P, Rieder E, et al.: Molecular and biological analysis of echovirus 9 strain isolated from a diabetic child. J Med Virol 2003, 69:529–537.
Titchener PA, Jenkins O, Szopa TM, et al.: Complete nucleotide sequence of a beta-cell tropic variant of coxsackievirus B4. J Med Virol 1994, 42:369–373.
Williams CH, Oikarinen S, Tauriainen S, et al.: Molecular analysis of an echovirus 3 strain isolated from an individual concurrently with appearance of islet cell and IA-2 autoantibodies. J Clin Microbiol 2006, 44:441–448.
Blomqvist S, Klemola P, Kaijalainen S, et al.: Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol 48:49–54.
Blomqvist S, Paananen A, Savolainen-Kopra C, et al.: Eight years of experience with molecular identification of human enteroviruses. J Clin Microbiol 2008, 46:2410–2413.
Palacios G, Oberste MS: Enteroviruses as agents of emerging infectious diseases. J Neurovirol 2005, 11:424–433.
Wikswo ME, Khetsuriani N, Fowlkes AL, et al.: Increased activity of Coxsackievirus B1 strains associated with severe disease among young infants in the United States, 2007–2008. Clin Infect Dis 2009, 49:e44–51.
Cabrerizo M, Echevarria JE, Otero A, et al.: Molecular characterization of a coxsackievirus A24 variant that caused an outbreak of acute haemorrhagic conjunctivitis in Spain, 2004. J Clin Virol 2008, 43:323–327.
Ding NZ, Wang XM, Sun SW, et al.: Appearance of mosaic enterovirus 71 in the 2008 outbreak of China. Virus Res 2009, 145:157–161.
Acknowledgment
This work is supported by grants from the European Union (EP7-HEALTH-2007, DIAPREPP N202013), the Juvenile Diabetes Research Foundation (USA), The Academy of Finland, and the SFB-TR19 from the Deutsche Forschungsgemeinschaft (DFG).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roivainen, M., Klingel, K. Virus Infections and Type 1 Diabetes Risk. Curr Diab Rep 10, 350–356 (2010). https://doi.org/10.1007/s11892-010-0139-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-010-0139-x
Keywords
- Type 1 diabetes
- Pancreatic islets
- β-Cell destruction
- Environmental factor
- Virus
- Enterovirus
- Coxsackievirus
- Echovirus
- Prolonged infection
- Chronic infection
- Enterovirus detection
- Detection of viral RNA
- In situ hybridization
- RT-PCR
- Immunostaining
- VP1 immunostaining
- Enterovirus 5-D8/1 antibody
- Diabetogenic mechanism
- Diabetogenic enteroviruses