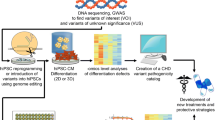
Abstract
Purpose of Review
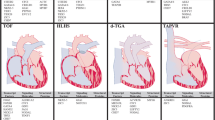
Congenital heart disease includes a wide variety of structural cardiac defects, the most severe of which are single ventricle defects (SVD). These patients suffer from significant morbidity and mortality; however, our understanding of the developmental etiology of these conditions is limited. Model organisms offer a window into normal and abnormal cardiogenesis yet often fail to recapitulate complex congenital heart defects seen in patients. The use of induced pluripotent stem cells (iPSCs) derived from patients with single-ventricle defects opens the door to studying SVD in patient-derived cardiomyocytes (iPSC-CMs) in a variety of different contexts, including organoids and chamber-specific cardiomyocytes. As the genetic and cellular causes of SVD are not well defined, patient-derived iPSC-CMs hold promise for uncovering mechanisms of disease development and serve as a platform for testing therapies. The purpose of this review is to highlight recent advances in iPSC-based models of SVD.
Recent Findings
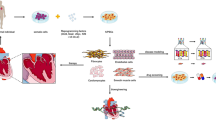
Recent advances in patient-derived iPSC-CM differentiation, as well as the development of both chamber-specific and non-myocyte cardiac cell types, make it possible to model the complex genetic and molecular architecture involved in SVD development. Moreover, iPSC models have become increasingly complex with the generation of 3D organoids and engineered cardiac tissues which open the door to new mechanistic insight into SVD development. Finally, iPSC-CMs have been used in proof-of-concept studies that the molecular underpinnings of SVD may be targetable for future therapies.
Summary
While each platform has its advantages and disadvantages, the use of patient-derived iPSC-CMs offers a window into patient-specific cardiogenesis and SVD development. Advancement in stem-cell based modeling of SVD promises to revolutionize our understanding of the developmental etiology of SVD and provides a tool for developing and testing new therapies.

Similar content being viewed by others
Abbreviations
- ASD:
-
Atrial septal defect
- CHD:
-
Congenital heart disease
- CM:
-
Cardiomyocyte
- HLHS:
-
Hypoplastic left heart syndrome
- iPSC:
-
Human-derived induced pluripotent stem cells
- LV:
-
Left ventricle
- PA/IVS:
-
Pulmonary atresia with intact ventricular septum
- SVD:
-
Single-ventricle disease
- RV:
-
Right ventricle
- TA:
-
Tricuspid atresia
- uAVCD:
-
Unbalanced atrioventricular canal defect
- VSD:
-
Ventricular septal defect
References
Papers of high interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44(1):138–43. https://doi.org/10.1016/j.jacc.2004.03.050.
van der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–7. https://doi.org/10.1016/j.jacc.2011.08.025.
van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011;8(1):50–60. https://doi.org/10.1038/nrcardio.2010.166.
Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck-Pringle D, Frommelt PC, Li JS, et al. Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137(21):2246–53. https://doi.org/10.1161/CIRCULATIONAHA.117.029375.
Foulks MG, Meyer RML, Gold JI, Herrington CS, Kallin K, Menteer J. Postoperative heart failure after stage 1 palliative surgery for single ventricle cardiac disease. Pediatr Cardiol. 2019;40(5):943–9. https://doi.org/10.1007/s00246-019-02093-4.
Michielon G, Parisi F, Di Carlo D, Squitieri C, Carotti A, Buratta M, et al. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg. 2003;24(4):502–10; discussion 10. https://doi.org/10.1016/s1010-7940(03)00342-7.
Garcia AM, Beatty JT, Nakano SJ. Heart failure in single right ventricle congenital heart disease: physiological and molecular considerations. Am J Physiol Heart Circ Physiol. 2020;318(4):H947–65. https://doi.org/10.1152/ajpheart.00518.2019.
Sano T, Ousaka D, Goto T, Ishigami S, Hirai K, Kasahara S, et al. Impact of cardiac progenitor cells on heart failure and survival in single ventricle congenital heart disease. Circ Res. 2018;122(7):994–1005. https://doi.org/10.1161/CIRCRESAHA.117.312311.
Fahed AC, Gelb BD, Seidman JG, Seidman CE. Genetics of congenital heart disease: the glass half empty. Circ Res. 2013;112(4):707–20. https://doi.org/10.1161/circresaha.112.300853.
Wilson RL, Troja W, Courtney J, Williams A, Jones HN. Placental and fetal characteristics of the Ohia mouse line recapitulate outcomes in human hypoplastic left heart syndrome. Placenta. 2022;117:131–8. https://doi.org/10.1016/j.placenta.2021.12.001.
Hofbauer P, Jahnel SM, Mendjan S. In vitro models of the human heart. Development. 2021;148(16). https://doi.org/10.1242/dev.199672.
Gabriel GC, Devine W, Redel BK, Whitworth KM, Samuel M, Spate LD, et al. Cardiovascular development and congenital heart disease modeling in the pig. Journal of the American Heart Association. 2021;10(14):e021631. https://doi.org/10.1161/jaha.121.021631.
Rahman A, DeYoung T, Cahill LS, Yee Y, Debebe SK, Botelho O, et al. A mouse model of hypoplastic left heart syndrome demonstrating left heart hypoplasia and retrograde aortic arch flow. Dis Model Mech. 2021;14(11). https://doi.org/10.1242/dmm.049077.
Shrestha R, Lieberth J, Tillman S, Natalizio J, Bloomekatz J. Using zebrafish to analyze the genetic and environmental etiologies of congenital heart defects. Adv Exp Med Biol. 2020;1236:189–223. https://doi.org/10.1007/978-981-15-2389-2_8.
Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun. 2017;8(1):1825. https://doi.org/10.1038/s41467-017-01946-x.
Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124(9):1631–42. https://doi.org/10.1242/dev.124.9.1631.
Ivanovitch K, Temiño S, Torres M. Live imaging of heart tube development in mouse reveals alternating phases of cardiac differentiation and morphogenesis. Elife. 2017;6. https://doi.org/10.7554/eLife.30668.
Kelly RG, Buckingham ME, Moorman AF. Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med. 2014;4(10). https://doi.org/10.1101/cshperspect.a015750.
Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91(6):457–69. https://doi.org/10.1161/01.res.0000034152.74523.a8.
Schmidt C, Deyett A, Ilmer T, Torres Caballer A, Jaendeler S, Pimpale L, et al. Multi-chamber cardioids unravel human heart development and cardiac defects. Biorxiv. 2022. https://doi.org/10.1101/2022.07.14.499699.
Erhardt S, Zheng M, Zhao X, Le TP, Findley TO, Wang J. The cardiac neural crest cells in heart development and congenital heart defects. J Cardiovasc Dev Dis. 2021;8(8). https://doi.org/10.3390/jcdd8080089.
Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105(5):408–21. https://doi.org/10.1161/CIRCRESAHA.109.201566.
Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, Riggs S, et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104(11):1567–73. https://doi.org/10.1172/jci8154.
Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126(6):1269–80.
Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18(3):271–5. https://doi.org/10.1038/ng0398-271.
Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18(3):266–70. https://doi.org/10.1038/ng0398-266.
Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–4. https://doi.org/10.1038/nature03940.
Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8(6):707–19. https://doi.org/10.1101/gad.8.6.707.
Togi K, Yoshida Y, Matsumae H, Nakashima Y, Kita T, Tanaka M. Essential role of Hand2 in interventricular septum formation and trabeculation during cardiac development. Biochem Biophys Res Commun. 2006;343(1):144–51. https://doi.org/10.1016/j.bbrc.2006.02.122.
Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16(2):154–60. https://doi.org/10.1038/ng0697-154.
Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270(5244):1995–9. https://doi.org/10.1126/science.270.5244.1995.
Vorisek CN, Zurakowski D, Tamayo A, Axt-Fliedner R, Siepmann T, Friehs I. Postnatal circulation in patients with aortic stenosis undergoing fetal aortic valvuloplasty: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2022;59(5):576–84. https://doi.org/10.1002/uog.24807.
Krane M, Dreßen M, Santamaria G, My I, Schneider CM, Dorn T, et al. Sequential defects in cardiac lineage commitment and maturation cause hypoplastic left heart syndrome. Circulation. 2021;144(17):1409–28. https://doi.org/10.1161/circulationaha.121.056198.
• Hinton RB, Martin LJ, Tabangin ME, Mazwi ML, Cripe LH, Benson DW. Hypoplastic left heart syndrome is heritable. J Am Coll Cardiol. 2007;50(16):1590–5. https://doi.org/10.1016/j.jacc.2007.07.021. This manuscript provides patient-derived iPSC-CM models of HLHS with thorough phenotypic characterization, providing valuable information on the pathogenesis of HLHS. The data presented further delineate intrinsic myocardial defects in SVD.
Zhu JY, Fu Y, Nettleton M, Richman A, Han Z. High throughput in vivo functional validation of candidate congenital heart disease genes in Drosophila. Elife. 2017;6. https://doi.org/10.7554/eLife.22617.
Schroeder AM, Allahyari M, Vogler G, Missinato MA, Nielsen T, Yu MS, et al. Model system identification of novel congenital heart disease gene candidates: focus on RPL13. Hum Mol Genet. 2019;28(23):3954–69. https://doi.org/10.1093/hmg/ddz213.
Theis JL, Vogler G, Missinato MA, Li X, Nielsen T, Zeng XI, et al. Patient-specific genomics and cross-species functional analysis implicate LRP2 in hypoplastic left heart syndrome. Elife. 2020;9. https://doi.org/10.7554/eLife.59554.
Akasaka T, Ocorr K, Lin L, Vogler G, Bodmer R, Grossfeld P. Overexpression of Kif1A in the developing Drosophila heart causes valvar and contractility defects: implications for human congenital heart disease. J Cardiovasc Dev Dis. 2020;7(2). https://doi.org/10.3390/jcdd7020022.
Fu Y, Yan W, Mohun TJ, Evans SM. Vertebrate tinman homologues XNkx2-3 and XNkx2-5 are required for heart formation in a functionally redundant manner. Development. 1998;125(22):4439–49. https://doi.org/10.1242/dev.125.22.4439.
Rotstein B, Paululat A. On the morphology of the Drosophila heart. J Cardiovasc Dev Dis. 2016;3(2). https://doi.org/10.3390/jcdd3020015.
Genge CE, Lin E, Lee L, Sheng X, Rayani K, Gunawan M, et al. The zebrafish heart as a model of mammalian cardiac function. Rev Physiol Biochem Pharmacol. 2016;171:99–136. https://doi.org/10.1007/112_2016_5.
Pfefferli C, Moran HR, Felker A, Mosimann C, Jazwinska A. Persistent ventricle partitioning in the adult zebrafish heart. J Cardiovasc Dev Dis. 2021;8(4). https://doi.org/10.3390/jcdd8040041.
Tu S, Chi NC. Zebrafish models in cardiac development and congenital heart birth defects. Differentiation. 2012;84(1):4–16. https://doi.org/10.1016/j.diff.2012.05.005.
Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, et al. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol Sci. 2008;106(1):193–205. https://doi.org/10.1093/toxsci/kfn154.
Gunawan F, Gentile A, Gauvrit S, Stainier DYR, Bensimon-Brito A. Nfatc1 promotes interstitial cell formation during cardiac valve development in zebrafish. Circ Res. 2020;126(8):968–84. https://doi.org/10.1161/circresaha.119.315992.
Ho S, Chan WX, Yap CH. Fluid mechanics of the left atrial ligation chick embryonic model of hypoplastic left heart syndrome. Biomech Model Mechanobiol. 2021;20(4):1337–51. https://doi.org/10.1007/s10237-021-01447-3.
Pesevski Z, Kvasilova A, Stopkova T, Nanka O, Drobna Krejci E, Buffinton C, et al. Endocardial fibroelastosis is secondary to hemodynamic alterations in the chick embryonic model of hypoplastic left heart syndrome. Dev Dyn. 2018;247(3):509–20. https://doi.org/10.1002/dvdy.24521.
Doetschman T, Azhar M. Cardiac-specific inducible and conditional gene targeting in mice. Circ Res. 2012;110(11):1498–512. https://doi.org/10.1161/CIRCRESAHA.112.265066.
Krishnan A, Samtani R, Dhanantwari P, Lee E, Yamada S, Shiota K, et al. A detailed comparison of mouse and human cardiac development. Pediatr Res. 2014;76(6):500–7. https://doi.org/10.1038/pr.2014.128.
Svensson EC, Huggins GS, Lin H, Clendenin C, Jiang F, Tufts R, et al. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding Fog-2. Nat Genet. 2000;25(3):353–6. https://doi.org/10.1038/77146.
Moazzen H, Wu Y, Engineer A, Lu X, Aulakh S, Feng Q. NOX2 is critical to endocardial to mesenchymal transition and heart development. Oxid Med Cell Longev. 2020;2020:1679045. https://doi.org/10.1155/2020/1679045.
Verma SK, Deshmukh V, Thatcher K, Belanger KK, Rhyner AM, Meng S, et al. RBFOX2 is required for establishing RNA regulatory networks essential for heart development. Nucleic Acids Res. 2022;50(4):2270–86. https://doi.org/10.1093/nar/gkac055.
Kathiriya IS, Rao KS, Iacono G, Devine WP, Blair AP, Hota SK, et al. Modeling human TBX5 haploinsufficiency predicts regulatory networks for congenital heart disease. Dev Cell. 2021;56(3):292–309e9. https://doi.org/10.1016/j.devcel.2020.11.020.
Firulli BA, Toolan KP, Harkin J, Millar H, Pineda S, Firulli AB. The HAND1 frameshift A126FS mutation does not cause hypoplastic left heart syndrome in mice. Cardiovasc Res. 2017;113(14):1732–42. https://doi.org/10.1093/cvr/cvx166.
Liu X, Yagi H, Saeed S, Bais AS, Gabriel GC, Chen Z, et al. The complex genetics of hypoplastic left heart syndrome. Nat Genet. 2017;49(7):1152–9. https://doi.org/10.1038/ng.3870.
Yagi H, Liu X, Gabriel GC, Wu Y, Peterson K, Murray SA, et al. The genetic landscape of hypoplastic left heart syndrome. Pediatr Cardiol. 2018;39(6):1069–81. https://doi.org/10.1007/s00246-018-1861-4.
Merklinger SL, Honjo O, Al-Radi OO, Poe J, Wang J, Oka N, et al. Primary in-series palliation of hypoplastic left heart syndrome with mechanical lung assist in neonatal pigs. ASAIO J. 2009;55(6):620–5. https://doi.org/10.1097/MAT.0b013e3181be00a0.
Wong FY, Veldman A, Sasi A, Teoh M, Edwards A, Chan Y, et al. Induction of left ventricular hypoplasia by occluding the foramen ovale in the fetal lamb. Sci Rep. 2020;10(1):880. https://doi.org/10.1038/s41598-020-57694-4.
Edwards A, Veldman A, Nitsos I, Chan Y, Brew N, Teoh M, et al. O052 model of hypoplastic left heart in the fetal lamb created using a percutaneous transhepatic catheter technique - preliminary experience. Global Heart. 2014;9(1, Supplement):e13. https://doi.org/10.1016/j.gheart.2014.03.1266.
Li Q, Hara H, Banks CA, Yamamoto T, Ayares D, Mauchley DC, et al. Anti-pig antibody in infants: can a genetically engineered pig heart bridge to allotransplantation? Ann Thorac Surg. 2020;109(4):1268–73. https://doi.org/10.1016/j.athoracsur.2019.08.061.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024.
Funakoshi S, Fernandes I, Mastikhina O, Wilkinson D, Tran T, Dhahri W, et al. Generation of mature compact ventricular cardiomyocytes from human pluripotent stem cells. Nat Commun. 2021;12(1):3155. https://doi.org/10.1038/s41467-021-23329-z.
Jiang Y, Habibollah S, Tilgner K, Collin J, Barta T, Al-Aama JY, et al. An induced pluripotent stem cell model of hypoplastic left heart syndrome (HLHS) reveals multiple expression and functional differences in HLHS-derived cardiac myocytes. Stem Cells Transl Med. 2014;3(4):416–23. https://doi.org/10.5966/sctm.2013-0105.
Hrstka SC, Li X, Nelson TJ. NOTCH1-dependent nitric oxide signaling deficiency in hypoplastic left heart syndrome revealed through patient-specific phenotypes detected in bioengineered cardiogenesis. Stem Cells. 2017;35(4):1106–19. https://doi.org/10.1002/stem.2582.
Kim MS, Fleres B, Lovett J, Anfinson M, Samudrala SSK, Kelly LJ, et al. Contractility of induced pluripotent stem cell-cardiomyocytes with an MYH6 head domain variant associated with hypoplastic left heart syndrome. Front Cell Dev Biol. 2020;8:440. https://doi.org/10.3389/fcell.2020.00440.
Yang C, Xu Y, Yu M, Lee D, Alharti S, Hellen N, et al. Induced pluripotent stem cell modelling of HLHS underlines the contribution of dysfunctional NOTCH signalling to impaired cardiogenesis. Hum Mol Genet. 2017;26(16):3031–45. https://doi.org/10.1093/hmg/ddx140.
Kobayashi J, Yoshida M, Tarui S, Hirata M, Nagai Y, Kasahara S, et al. Directed differentiation of patient-specific induced pluripotent stem cells identifies the transcriptional repression and epigenetic modification of NKX2–5, HAND1, and NOTCH1 in hypoplastic left heart syndrome. PLoS One. 2014;9(7):e102796. https://doi.org/10.1371/journal.pone.0102796.
Paige SL, Galdos FX, Lee S, Chin ET, Ranjbarvaziri S, Feyen DAM, et al. Patient-specific induced pluripotent stem cells implicate intrinsic impaired contractility in hypoplastic left heart syndrome. Circulation. 2020;142(16):1605–8. https://doi.org/10.1161/circulationaha.119.045317.
Xu X, Jin K, Bais AS, Zhu W, Yagi H, Feinstein TN, et al. Uncompensated mitochondrial oxidative stress underlies heart failure in an iPSC-derived model of congenital heart disease. Cell Stem Cell. 2022;29(5):840-55.e7. https://doi.org/10.1016/j.stem.2022.03.003.
Xu X, Lin JI, Bais AS, Reynolds MJ, Tan T, Gabriel GC, et al. Mitochondrial respiration defects in single-ventricle congenital heart disease. Front Cardiovasc Med. 2021;8:734388. https://doi.org/10.3389/fcvm.2021.734388.
Chapman G, Moreau JLM, Ip E, Szot JO, Iyer KR, Shi H, et al. Functional genomics and gene-environment interaction highlight the complexity of congenital heart disease caused by Notch pathway variants. Human Molecular Genetics. 2019;29(4):566–79. https://doi.org/10.1093/hmg/ddz270.
Miao Y, Tian L, Martin M, Paige SL, Galdos FX, Li J, et al. Intrinsic endocardial defects contribute to hypoplastic left heart syndrome. Cell Stem Cell. 2020;27(4):574–89e8. https://doi.org/10.1016/j.stem.2020.07.015.
Knight WE, Cao Y, Lin YH, Chi C, Bai B, Sparagna GC, et al. Maturation of pluripotent stem cell-derived cardiomyocytes enables modeling of human hypertrophic cardiomyopathy. Stem Cell Reports. 2021;16(3):519–33. https://doi.org/10.1016/j.stemcr.2021.01.018.
McKeithan WL, Savchenko A, Yu MS, Cerignoli F, Bruyneel AAN, Price JH, et al. An automated platform for assessment of congenital and drug-induced arrhythmia with hiPSC-derived cardiomyocytes. Front Physiol. 2017;8:766. https://doi.org/10.3389/fphys.2017.00766.
Yoshinaga D, Baba S, Makiyama T, Shibata H, Hirata T, Akagi K, et al. Phenotype-based high-throughput classification of long QT syndrome subtypes using human induced pluripotent stem cells. Stem Cell Reports. 2019;13(2):394–404. https://doi.org/10.1016/j.stemcr.2019.06.007.
Del Alamo JC, Lemons D, Serrano R, Savchenko A, Cerignoli F, Bodmer R, et al. High throughput physiological screening of iPSC-derived cardiomyocytes for drug development. Biochim Biophys Acta. 2016;1863(7 Pt B):1717–27. https://doi.org/10.1016/j.bbamcr.2016.03.003.
Stillitano F, Hansen J, Kong CW, Karakikes I, Funck-Brentano C, Geng L, et al. Modeling susceptibility to drug-induced long QT with a panel of subject-specific induced pluripotent stem cells. Elife. 2017;6. https://doi.org/10.7554/eLife.19406.
Scherba JC, Karra R, Turek JW, Bursac N. Toward improved understanding of cardiac development and congenital heart disease: the advent of cardiac organoids. J Thorac Cardiovasc Surg. 2022. https://doi.org/10.1016/j.jtcvs.2022.02.028.
Kahn-Krell A, Pretorius D, Guragain B, Lou X, Wei Y, Zhang J, et al. A three-dimensional culture system for generating cardiac spheroids composed of cardiomyocytes, endothelial cells, smooth-muscle cells, and cardiac fibroblasts derived from human induced-pluripotent stem cells. Front Bioeng Biotechnol. 2022;10:908848. https://doi.org/10.3389/fbioe.2022.908848.
Campostrini G, Meraviglia V, Giacomelli E, van Helden RWJ, Yiangou L, Davis RP, et al. Generation, functional analysis and applications of isogenic three-dimensional self-aggregating cardiac microtissues from human pluripotent stem cells. Nat Protoc. 2021;16(4):2213–56. https://doi.org/10.1038/s41596-021-00497-2.
• Marini V, Marino F, Aliberti F, Giarratana N, Pozzo E, Duelen R, et al. Long-term culture of patient-derived cardiac organoids recapitulated Duchenne muscular dystrophy cardiomyopathy and disease progression. Front Cell Dev Biol. 2022;10:878311. https://doi.org/10.3389/fcell.2022.878311. This manuscript outlines a high-throughput approach to cardiac organoid creation, and models the pathogenesis of diabetes- associated congenital heart disease with a cardiac organoid platform. Though not reflective of single- ventricle disease, this manuscript represents a step towards high- fidelity CHD modeling with cardiac organoids.
Lewis-Israeli YR, Wasserman AH, Gabalski MA, Volmert BD, Ming Y, Ball KA, et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun. 2021;12(1):5142. https://doi.org/10.1038/s41467-021-25329-5.
Hoang P, Kowalczewski A, Sun S, Winston TS, Archilla AM, Lemus SM, et al. Engineering spatial-organized cardiac organoids for developmental toxicity testing. Stem Cell Reports. 2021;16(5):1228–44. https://doi.org/10.1016/j.stemcr.2021.03.013.
Jackman CP, Ganapathi AM, Asfour H, Qian Y, Allen BW, Li Y, et al. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials. 2018;159:48–58. https://doi.org/10.1016/j.biomaterials.2018.01.002.
Drakhlis L, Biswanath S, Farr CM, Lupanow V, Teske J, Ritzenhoff K, et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol. 2021;39(6):737–46. https://doi.org/10.1038/s41587-021-00815-9.
Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3(12). https://doi.org/10.1172/jci.insight.99941.
Kaushal S, Hare JM, Shah AM, Pietris NP, Bettencourt JL, Piller LB, et al. Autologous cardiac stem cell injection in patients with hypoplastic left heart syndrome (CHILD study). Pediatr Cardiol. 2022. https://doi.org/10.1007/s00246-022-02872-6.
Burkhart HM, Qureshi MY, Rossano JW, Cantero Peral S, O’Leary PW, Hathcock M, et al. Autologous stem cell therapy for hypoplastic left heart syndrome: safety and feasibility of intraoperative intramyocardial injections. J Thorac Cardiovasc Surg. 2019;158(6):1614–23. https://doi.org/10.1016/j.jtcvs.2019.06.001.
Hrstka SC, Li X, Nelson TJ, Group WPGP. NOTCH1-dependent nitric oxide signaling deficiency in hypoplastic left heart syndrome revealed through patient-specific phenotypes detected in bioengineered cardiogenesis. Stem Cells. 2017;35(4):1106–19. https://doi.org/10.1002/stem.2582.
Funding
LP is supported by the Duke University School of Medicine Medical Scientist Training Program (T32-GM-145449–01). APL is supported by the National Institutes of Health (K08-HL136839 and R01-HL160654), Doris Duke Charitable Foundation (CSDA-2020098), and Additional Ventures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Regenerative Medicine
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parker, L.E., Kurzlechner, L.M. & Landstrom, A.P. Induced Pluripotent Stem Cell–Based Modeling of Single-Ventricle Congenital Heart Diseases. Curr Cardiol Rep 25, 295–305 (2023). https://doi.org/10.1007/s11886-023-01852-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-01852-3