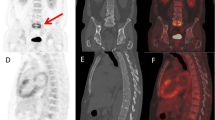
Abstract
Purpose of Review
Cardiac sarcoidosis (CS) is an inflammatory disease of unknown etiology that can lead to life-threatening arrhythmias, heart failure, and death. Advanced cardiac imaging modalities have improved the clinician’s ability to detect this disease. The purpose of this review is to discuss the recent evidence of cardiac metabolic imaging as assessed by [18F]FDG PET and [123I]BMIPP SPECT in the evaluation of CS patients.
Recent Findings
[18F]FDG PET is the gold standard to identify myocardial inflammation. [123I]BMIPP SPECT can uncover early myocardial damage as well as advanced stages of CS when fibrosis prevails. In presence of inflammation, myocardial [18F]FDG uptake is increased, but in contrast, BMIPP myocardial uptake is reduced or even suppressed. Thus, a complementary role of cardiac metabolic imaging by [18F]FDG PET and BMIPP SPECT has been proposed to detect the whole spectrum of CS.
Summary
[18F]FDG PET is considered an important tool to improve the diagnosis and optimize the management of CS. The role of [123I]BMIPP SPECT in diagnosing CS is still under investigation. Further studies are needed to evaluate the clinical utility of combined cardiac metabolic imaging in the diagnosis, prognosis, and for selecting treatments in CS patients.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Slart RHJA, Glaudemans AWJM, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, et al. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol. 2018;25(1):298–319. https://doi.org/10.1007/s12350-017-1043-4.
Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119(2):167–72.
Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–41. https://doi.org/10.1093/oxfordjournals.aje.a009096.
• Bokhari S, Sheikh T. Cardiac sarcoidosis: advantages and limitations of advanced cardiac imaging. J Nucl Cardiol. 2021. https://doi.org/10.1007/s12350-021-02684-w. This editorial mainly addresses how advanced cardiac imaging modalities can be utilized in the diagnosis and management of cardiac sarcoidosis.
Blankstein R, Chareonthaitawee P. Patient with known or suspected cardiac sarcoidosis. In: Di Carli MF, editor. Nuclear cardiology and multimodal cardiovascular imaging: a companion to Braunwald’s heart disease. Philadelphia, Elsevier, 2022.
•• Bravo PE, Singh A, Di Carli MF, Blankstein R. Advanced cardiovascular imaging for the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2019;26(1):188–99. https://doi.org/10.1007/s12350-018-01488-9. This review highlights that despite significant recent developments, the diagnosis of cardiac sarcoidosis remains challenging. Nevertheless, FDG-PET and CMR-LGE provide complementary information for the management of cardiac sarcoidosis.
Wiefels C, Lamai O, Kandolin R, Birnie D, Leung E, Tinoco Mesquita C, et al. The role of 18F-FDG-PET/CT in cardiac sarcoidosis. Int J Cardiovasc Sci. 2020;33:389–400. https://doi.org/10.36660/ijcs.20200033.
Trivieri MG, Spagnolo P, Birnie D, Liu P, Drake W, Kovacic JC, et al. Challenges in cardiac and pulmonary sarcoidosis. J Am Coll Cardiol. 2020;76(16):1878–901. https://doi.org/10.1016/j.jacc.2020.08.042.
Diagnostic standard and guidelines for sarcoidosis [in Japanese]. Japanese J Sarcoidosis Other Granulomatous Disord. 2007;27:89–102.
Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, et al. JCS 2016 Guidelines for the diagnosis and treatment of cardiac sarcoidosis – digest version. Circ J. 2019;83(11):2329–88. https://doi.org/10.1253/circj.CJ-19-0508.
Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–23. https://doi.org/10.1016/j.hrthm.2014.03.043.
•• Kron J, Crawford T. The cardiac sarcoidosis consortium: elucidating a mysterious disease through collaborative research. Eur Heart J. 2022; 1–3. https://doi.org/10.1093/eurheartj/ehac358. Online ahead of print. This paper emphasizes the creation of the cardiac sarcoidosis consortium and its goals.
Kaminaga T, Takeshita T, Yamauchi T, Kawamura H, Yasuda M. The role of Iodine-123-labeled 15-(p-iodophenyl)-3R, S-methylpentadecanoic acid scintigraphy in the detection of focal myocardial involvement of sarcoidosis. Int J Cardiol. 2004;94(1):99–103. https://doi.org/10.1016/j.ijcard.2003.05.012.
Momose M, Fukushima K, Kondo C, Serizawa N, Suzuki A, Abe K, et al. Diagnosis and detection of myocardial injury in active cardiac sarcoidosis –Significance of myocardial fatty acid metabolism and myocardial perfusion mismatch. Circ J. 2015;79(12):2669–76. https://doi.org/10.1253/circj.CJ-15-0681.
Yoshinaga K. Role of metabolic imaging in detecting cardiac involvement in sarcoidosis. Circ J. 2015;79:2551–2. https://doi.org/10.1253/circj.CJ-15-1099.
Manabe O, Tamaki N. Potential roles of 123I-BMIPP SPECT to assess cardiac sarcoidosis. J Nucl Cardiol. 2021;28(3):936–8.
•• Yamamoto A, Nagao M, Watanabe E, Inamura Y, Suzuki A, Fukushima K, et al. Prognosis and recurrence in cardiac sarcoidosis: serial assessment of BMIPP-SPECT and FDG-PET. J Nucl Cardiol 2021;28(3):919–29. https://doi.org/10.1007/s12350-021-02567-0. This study showed for the first time that BDS was a predictive marker of recurrence and MACE in cardiac sarcoidosis patients.
Campisi R, Merani MF, Marina MI. BMIPP SPECT in cardiac sarcoidosis: a marker of risk? J Nucl Cardiol. 2021;28(3):930–5. https://doi.org/10.1007/s12350-021-02626-6.
Bois JP, Muser D, Chareonthaitawee P. PET/CT evaluation of cardiac sarcoidosis. PET Clin. 2019;14(2):223–32. https://doi.org/10.1016/j.cpet.2018.12.004.
Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9:e000867. https://doi.org/10.1161/CIRCIMAGING.113.000867.
Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, et al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG-PET/CT in cardiac sarcoid detection and therapy monitoring writing group. J Nucl Med. 2017;58(8):1341–53. https://doi.org/10.2967/jnumed.117.196287.
Chikamori T, Fujita H, Nanasato M, Toba M, Nishimura T. Prognostic value of I-123 15-(p-iodophenyl)-3-(R, S) methylpentadecanoic acid myocardial imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2005;12(2):172–8. https://doi.org/10.1016/j.nuclcard.2004.12.293.
Tamaki N, Yoshinaga K. Novel iodinated tracers, MIBG and BMIPP for nuclear cardiology. J Nucl Cardiol. 2011;18(1):135–43. https://doi.org/10.1007/s12350-010-9305-4.
Kudo T, Myssayev A, Ideguchi R. Recent advances in BMIPP Imaging. In Shinro Matsuo, editor. Clinical nuclear cardiology: practical applications and future directions (frontiers in myocardia). Bentham Science Publishers; 2018.
Dilsizian V, Bateman TM, Bergmann SR, Des Prez R, Magram MY, Goodbody AE, et al. Metabolic imaging with beta-methyl-p-[(123)I]-iodophenyl-pentadecanoic acid identifies ischemic memory after demand ischemia. Circulation. 2005;112(14):2169–74. https://doi.org/10.1161/CIRCULATIONAHA.104.530428.
Kontos MC, Dilsizian V, Weiland F, DePuey G, Mahmarian JJ, Iskandrian AE, et al. Iodofiltic acid I 123 (BMIPP) fatty acid imaging improves initial diagnosis in emergency department patients with suspected acute coronary syndromes: a multicenter trial. J Am Coll Cardiol. 2010;56(4):290–9. https://doi.org/10.1016/j.jacc.2010.03.045.
Schindler TH, Bateman TM, Berman DS, Chareonthaitawee P, De Blanche LE, Dilsizian V, et al. Appropriate use criteria for PET myocardial perfusion imaging. J Nucl Med. 2020;61(8):1221–65. https://doi.org/10.2967/jnumed.120.246280.
• Saric P, Young KA, Rodriguez-Porcel M, Chareonthaitawee P. PET imaging in cardiac sarcoidosis: a narrative review with focus on novel PET tracers. Pharmaceuticals (Basel). 2021;14(12):1286. https://doi.org/10.3390/ph14121286. This review summarizes several aspects of the current use of PET in cardiac sarcoidosis. Additionally, the review discusses novel PET radiotracers of potential interest in cardiac sarcoidosis.
Bokhari S, Lin JC, Julien HM. FDG-PET is a superior tool in the diagnosis and management of cardiac sarcoidosis. J Am Coll Cardiol. 2017. Expert analysis.
Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21(5):925–39. https://doi.org/10.1007/s12350-014-9901-9.
Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC. Reduction in (18)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2013;21(1):166–74. https://doi.org/10.1007/s12350-013-9828-6.
Waller AH, Blankstein R. Quantifying myocardial inflammation using 18F-fluorodeoxyglucose positron emission tomography in cardiac sarcoidosis. J Nucl Cardiol. 2014;21(5):940–3. https://doi.org/10.1007/s12350-014-9921-5.
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR. 2010;195(2):310–20. https://doi.org/10.2214/AJR.10.4923.
Kataoka S, Momose M, Fukushima K, Serizawa N, Suzuki A, Kondo C, et al. Regional myocardial damage and active inflammation in patients with cardiac sarcoidosis detected by non-invasive multi-modal imaging. Ann Nucl Med. 2017;31(2):135–43. https://doi.org/10.1007/s12149-016-1136-1.
Kudo T. Present status of medical radiation and nuclear cardiology usage in Japan: a discussion at the American Society of Nuclear Cardiology Joint Symposium. Ann Nucl Cardiol. 2018;4(1):142–148. https://doi.org/10.17996/anc.18-00071.
He Z-X, Shi R-F, Wu Y-J, Tian Y-Q, Liu X-J, Wang S-W, et al. Direct imaging of exercise-induced myocardial ischemia with fluorine-18–labeled deoxyglucose and Tc-99m-sestamibi in coronary artery disease. Circulation. 2003;108:1208–13. https://doi.org/10.1161/01.CIR.0000088784.25089.D9.
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–226. https://doi.org/10.1007/s12350-016-0522-3.
Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53(2):241–8. https://doi.org/10.2967/jnumed.111.090662.
Kim SJ, Pak K, Kim K. Diagnostic performance of F-18 FDG PET for detection of cardiac sarcoidosis; a systematic review and meta-analysis. J Nucl Cardiol. 2020;27(6):2103–15. https://doi.org/10.1007/s12350-018-01582-y.
Tang R, Wang JTY, Wang L, et al. Impact of patient preparation on the diagnostic performance of 18F-FDG PET in cardiac sarcoidosis: a systematic review and meta-analysis. Clin Nucl Med. 2016;41(7):e327–39. https://doi.org/10.1097/RLU.0000000000001063.
•• Aitken M, Chan MV, Urzua Fresno C, Farrell A, Islam N, McInnes MDF, et al. Diagnostic accuracy of cardiac MRI versus FDG PET for cardiac sarcoidosis: a systematic review and meta-analysis. Radiology. 2022;304(3):556–79. https://doi.org/10.1148/radiol.213170. This systematic review and meta-analysis showed that cardiac MRI has a higher sensitivity than FDG PET for diagnosis of cardiac sarcoidosis but similar specificity.
Torizuka K, Yonekura Y, Nishimura T, Tamaki N, Uehara T, Ikekubo K, et al. A phase 1 study of beta-methyl-p-(123I)-iodophenyl-pentadecanoic acid (123I-BMIPP) (article in Japanese). Kaku Igaku. 1991;28(7):681–90.
Kurata C, Tawarahara K, Taguchi T, Aoshima S, Kobayashi A, Yamazaki N, et al. Myocardial emission computed tomography with iodine-123-labeled beta-methyl-branched fatty acid in patients with hypertrophic cardiomyopathy. J Nucl Med. 1992;33(1):6–13.
Matsushita T, Ikeda S, Iwasaki K. Utility of {sup123} I-{beta}-methyl iodophenyl pentadecanoic acid (BMIPP) myocardial scintigraphy in the diagnosis of the cardiac involvement in cardiac sarcoidosis. Nippon Kyobu Rinsho (Jpn J Chest Dis.) 1999;58.
Matsumoto K, Ehara S, Sakaguchi M, Otsuka K, Hasegawa T, Shimada K, et al. Clinical characteristics of late gadolinium enhancement in patients with cardiac sarcoidosis. Osaka City Med J. 2015;61(1):9–17.
Yamamoto A, Nagao M, Fukushima K, Ando K, Nakao R, Goto M, et al. Adverse cardiac events in cardiac sarcoidosis: prediction by BMIPP-SPECT and CMR LGE. Am J Cardiol. 2022;180:149–54. https://doi.org/10.1016/j.amjcard.2022.06.040.
Tsujimura E, Kusuoka H, Fukuchi K, Hasegawa S, Yutani K, Hori M, et al. Changes in perfusion and fatty acid metabolism of rat heart with autoimmune myocarditis. Ann Nucl Med. 2000;14(5):361–7. https://doi.org/10.1007/BF02988696.
Bravo PE, Taqueti VR. Cardiac MRI vs. PET for the evaluation of cardiac sarcoidosis: consider MRI. Am Coll Cardiol. 2017. Expert analysis.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy V, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329–36. https://doi.org/10.1016/j.jacc.2013.09.022.
• Ahmed I, Abebe AT, Han Y, Alnabelsi T, Agrawal T, Kassi M, et al. The prognostic role of cardiac positron emission tomography imaging in patients with sarcoidosis: a systematic review. J Nucl Cardiol. 2021;24(4):1545–52. https://doi.org/10.1007/s12350-021-02681-z. This review summarizes the current evidence of the prognostic value of PET in cardiac sarcoidosis that involves 6 studies.
Flores RJ, Flaherty KR, Jin Z, Bokhari S. The prognostic value of quantitating and localizing F-18 FDG uptake in cardiac sarcoidosis. J Nucl Cardiol. 2020;27(6):2003–10. https://doi.org/10.1007/s12350-018-01504-y.
Muser D, Santangeli P, Castro SA, Liang JJ, Enriquez A, Werner TJ, et al. Prognostic value of serial quantitative evaluation of (18)F-fluoro-deoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging. 2018;45(8):1394–404. https://doi.org/10.1007/s00259-018-4001-8.
Sperry BW, Tamarappoo BK, Oldan JD, Javed O, Culver DA, Brunken R, et al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on 18F-FDG scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11(2 Pt 2):336–45. https://doi.org/10.1016/j.jcmg.2017.04.020.
Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6(4):501–11. https://doi.org/10.1016/j.jcmg.2012.10.021.
Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120(20):1969–77. https://doi.org/10.1161/CIRCULATIONAHA.109.851352.
Hulten E, Agarwal V, Cahill M, et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9(9):e005001. https://doi.org/10.1161/CIRCIMAGING.116.005001.
Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29(9):1034–41. https://doi.org/10.1016/j.cjca.2013.02.004.
JCS Joint Working Group. Guidelines for clinical use of cardiac nuclear medicine (JCS 2010) – digest version. Circ J. 2012;76(3):761–7. https://doi.org/10.1253/circj.cj-88-0019.
Acknowledgements
The authors are thankful to Daniel Cirigliano for the artwork and to Dr. Claudia Cortés for the thoughtful comments that strengthened this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Campisi, R., Merani, M.F. & Rodríguez, M.I. Assessment of Cardiac Sarcoidosis: FDG PET and BMIPP SPECT. Curr Cardiol Rep 24, 1873–1882 (2022). https://doi.org/10.1007/s11886-022-01803-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01803-4