Abstract
Purpose of Review
Coronary artery disease (CAD) is the leading contributor to cardiovascular disease; it is the most prevalent non-communicable disease globally and has high morbidity, mortality and health care cost. Risk stratification is defined as prevention or containment of disease prior to it occurring or progressing, and non-invasive surrogates include history, examination, biomarkers and non-invasive imaging. This review aims to highlight advancement in current diagnostic strategies and explores gaps for CAD secondary to atherosclerosis and non-obstructive vascular diseases.
Recent Findings
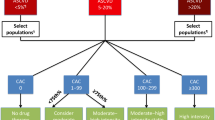
Cardiac risk scores have largely proven inadequate in risk stratifying heterogeneous patient populations. Greater emphasis should also be provided to posttest risk stratification. Non-invasive imaging with MRI is the most accurate but least cost efficacious presently due to availability and expertise. Echocardiography and nuclear imaging have good accuracy, but radiation limits the latter. Novel echocardiographic technologies may increase its appeal. Cardiac CT angiography is increasingly promising.
Summary
Non-invasive and minimally invasive imaging has significantly influenced the cost-efficacy trajectory of coronary artery disease diagnosis and management. Recent studies suggest that future guidelines will incorporate more subclassifications from the findings of these novel technologies and for more diverse patient demographics.

Similar content being viewed by others
Notes
Non-invasive strategies in this review incorporate risk scores, functional and anatomical information on CAD; imaging utilizing injected contrast, radioactive isotopes or drugs labelled ‘minimally invasive’ are also included.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Iyngkaran P, Chan W, Liew D, Zamani J, Horowitz JD, Jelinek M, et al. Risk stratification for coronary artery disease in multi-ethnic populations: Are there broader considerations for cost efficiency? World J Methodol. 2019;9(1):1–19.
Joseph J, Velasco A, Hage FG, Reyes E. Comparison of ESC and ACC/AHA guidelines for the diagnosis and management of patients with stable coronary artery disease. J Nucl Cardiol. 2018;25(2):509–15. https://doi.org/10.1007/s12350-017-1055-0.
Buccheri S, D’Arrigo P, Franchina G, Capodanno D. Risk stratification in patients with coronary artery disease: A practical walkthrough in the landscape of prognostic risk models. Intervent Cardiol Rev. 2018;13(3):112–20. https://doi.org/10.15420/icr.2018.16.2.
Omland T, White HD. Blood biomarkers for risk stratification in patients with stable ischemic heart disease. Clin Chem. 2017;63:1.
Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. American College of Cardiology Foundation Appropriate Use Criteria Task ForceACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63(4):380–406. https://doi.org/10.1016/j.jacc.2013.11.009.
Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130(19):1749–67. https://doi.org/10.1161/CIR.0000000000000095.
Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. Guidelines ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–41.
Skelly AC, Hashimoto R, Buckley DI, et al. Noninvasive testing for coronary artery disease [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); (Comparative Effectiveness Reviews, No. 171.) Introduction. 2016. Available from: https://www.ncbi.nlm.nih.gov/books/NBK361133/
Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. https://doi.org/10.1093/eurheartj/eht296.
Foldyna B, Udelson JE, Karády J, Banerji D, Lu MT, Mayrhofer T, et al. Pretest probability for patients with suspected obstructive coronary artery disease: re-evaluating Diamond-Forrester for the contemporary era and clinical implications: insights from the PROMISE trial. Eur Heart J Cardiovasc Imaging. 2018. https://doi.org/10.1093/ehjci/jey182.
Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1:574–5.
Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation. 2011;124(22):2423–32, 1-8. https://doi.org/10.1161/CIRCULATIONAHA.111.039255.
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–300. https://doi.org/10.1056/NEJMoa1415516.
Dancy L, O'Gallagher K, Milton P, Sado D. New NICE guidelines for the management of stable angina. Br J Gen Pract. 2018;68(669):202–3. https://doi.org/10.3399/bjgp18X695693.
Carrabba N, Migliorini A, Pradella S, Acquafresca M, Guglielmo M, Baggiano A, et al. Old and New NICE guidelines for the evaluation of new onset stable chest pain: a real world perspective. BioMed Res Int. 2018. https://doi.org/10.1155/2018/3762305.
Genders TS, Petersen SE, Pugliese F, Dastidar AG, Fleischmann KE, Nieman K, et al. The optimal imaging strategy for patients with stable chest pain: a cost-effectiveness analysis. Ann Intern Med. 2015;162:474–84. https://doi.org/10.7326/M14-0027.
Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, et al. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation. 2017;136(21):1993–2005. https://doi.org/10.1161/CIRCULATIONAHA.117.030578.
SCOT-HEART Investigators, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–33. https://doi.org/10.1056/NEJMoa1805971.
•• Adamson PD, Newby DE, Hill CL, Coles A, Douglas PS, Fordyce CB. Comparison of international guidelines for assessment of suspected stable angina: insights from the PROMISE and SCOT-HEART. JACC Cardiovasc Imaging. 2018;11(9):1301–10. https://doi.org/10.1016/j.jcmg.2018.06.021 Excellent analysis of advancements in coronary artery imaging.
Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–8.
Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–906.
Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53(4):323–30. https://doi.org/10.1016/j.jacc.2008.08.074.
Michelsen MM, Mygind ND, Pena A, Aziz A, Frestad D, Høst N, et al. Peripheral reactive hyperemia index and coronary microvascular function in women with no obstructive CAD: the iPOWER Study. JACC Cardiovasc Imaging. 2016;9:411–7.
Knuuti, J, Ballo H, Juarez-Orozco LE, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. 2018.
Siontis GCM, Mavridis D, Greenwood JP, Coles B, Nikolakopoulou A, Jüni P, et al. Outcomes of non-invasive diagnostic modalities for the detection of coronary artery disease: network meta-analysis of diagnostic randomised controlled trials. BMJ. 2018;360:k452. https://doi.org/10.1136/bmj.k452.
Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J, et al. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation. 2014;129(10):1129–38. https://doi.org/10.1161/CIRCULATIONAHA.112.000071.
Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al. MR-IMPACT II: magnetic resonance imaging for myocardial perfusion assessment in coronary artery disease trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34(10):775–81. https://doi.org/10.1093/eurheartj/ehs022.
•• SCOT-HEART Investigators, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–33. https://doi.org/10.1056/NEJMoa1805971 Landmark trial on coronary artery imaging.
Metz LD, Beattie M, Hom R, et al. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007;49:227–37.
Gaibazzi N, Reverberi C, Lorenzoni V, et al. Prognostic value of high-dose dipyridamole stress myocardial contrast perfusion echo- cardiography. Circulation. 2012;126:1217–24.
Dogdus M, Simsek E, Cinar CS. 3D-speckle tracking echocardiography for assessment of coronary artery disease severity in stable angina pectoris. Echocardiography. 2019;36:320–7.
Kaufmann PA, et al. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102(11):1233–8.
Dayanikli F, et al. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994;90(2):808–17.
Gould KL, et al. Short-term cholesterol lowering decreases size and severity of perfusion abnormalities by positron emission tomography after dipyridamole in patients with coronary artery disease. A potential noninvasive marker of healing coronary endothelium. Circulation. 1994;89(4):1530–8.
Kaufmann PA, et al. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J Am Coll Cardiol. 2000;36(1):103–9.
Nitenberg A, et al. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42(7):1017–25.
Yokoyama I, et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30(6):1472–7.
Di Carli MF, et al. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41(8):1387–93.
Schindler TH, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47(6):1188–95.
Olsen MH, et al. Association between vascular dysfunction and reduced myocardial flow reserve in patients with hypertension: a LIFE substudy. J Hum Hypertens. 2004;18(6):445–52.
Akinboboye OO, Chou RL, Bergmann SR. Augmentation of myocardial blood flow in hypertensive heart disease by angiotensin antagonists: a comparison of lisinopril and losartan. J Am Coll Cardiol. 2002;40(4):703–9.
Stapleton PA, et al. Hypercholesterolemia and microvascular dysfunction: interventional strategies. J Inflamm (Lond). 2010;7:54.
Pitkanen OP, et al. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47(2):248–54.
Kemp HG Jr. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32(3):375–6.
Cannon RO III. Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol. 2009;54(10):877–85.
Douglas PS, et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58(8):801–9.
Jespersen L, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–44.
Ong P, et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59(7):655–62.
Epstein SE, Cannon RO III. Site of increased resistance to coronary flow in patients with angina pectoris and normal epicardial coronary arteries. J Am Coll Cardiol. 1986;8(2):459–61.
Epstein SE, Cannon RO III, Bonow RO. Exercise testing in patients with microvascular angina. Circulation. 1991;83(5 Suppl):III73–6.
Shaw LJ, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114(9):894–904.
Halcox JP, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–8.
von Mering GO, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109(6):722–5.
Johnson BD, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27(12):1408–15.
Pepine CJ, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–32.
Thomson LE, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4).
Kaski JC, et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25(4):807–14.
Lamendola P, et al. Long-term prognosis of patients with cardiac syndrome X. Int J Cardiol. 2010;140(2):197–9.
Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121(21):2317–25.
Gulati M, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169(9):843–50.
Taqueti VR, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131(6):528–35.
Cook CM, et al. Association between physiological stenosis severity and angina-limited exercise time in patients with stable coronary artery disease. JAMA Cardiol. 2019. https://doi.org/10.1001/jamacardio.2019.1139.
Siontis GCM, Mavridis D, Greenwood JP, Coles B, Nikolakopoulou A, Juni P, et al. Outcomes of non-invasive diagnostic modalities for the detection of coronary artery disease: network meta-analysis of diagnostic randomised controlled trials. BMJ. 2018;360:k452. https://doi.org/10.1136/bmj.k452.
Cohn JN, Quyyumi AA, Hollenberg MK, Jamerson KA. Surrogate markers for cardiovascular disease functional markers. Circulation. 2004;109[suppl IV:IV-31–46. https://doi.org/10.1161/01.CIR.0000133442.99186.39.
Feinstein SB, Voci P, Pizzuto F. Noninvasive surrogate markers of atherosclerosis. Am J Card. 2002;89(5):31–43.
Upadhyay RK. Emerging risk biomarkers in cardiovascular diseases and disorders. J Lipids. 2015. https://doi.org/10.1155/2015/971453.
Garg N, Muduli SK, Kapoor A, Tewari S, Kumar S, Khanna R, et al. Comparison of different cardiovascular risk score calculators for cardiovascular risk prediction and guideline recommended statin uses. Indian Heart J. 2017;69(4):458–63. https://doi.org/10.1016/j.ihj.2017.01.015.
Anand SS, Yusuf S, Vuksan V, et al. Difference in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet. 2000;356:279–84.
Vaduganathan M, Rao RS, Koschinsky M, et al. Evaluation of Lp (a) and other independent risk factors for CHD in Asian Indians and their USA counterparts. J Lipid Res. 2001;42:631–8.
Iyngkaran P, Liew D, McDonald P, Thomas MC, Reid C, Chew D, et al. Phase 4 studies in heart failure - what is done and what is needed? Curr Cardiol Rev. 2016;12(3):216–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
P. Iyngkaran, S. Noaman, W. Chan, G. Mahadavan, M.C. Thomas and S. Rajendran have won independent and governmental research funding. None pose a conflict of interest for this review.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiac PET, CT, and MRI
Rights and permissions
About this article
Cite this article
Iyngkaran, P., Noaman, S., Chan, W. et al. Non-invasive Risk Stratification for Coronary Artery Disease: Is It Time for Subclassifications?. Curr Cardiol Rep 21, 87 (2019). https://doi.org/10.1007/s11886-019-1174-0
Published:
DOI: https://doi.org/10.1007/s11886-019-1174-0