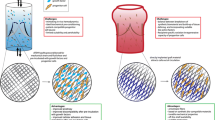
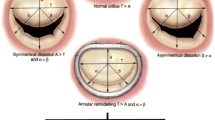
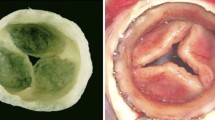
Abstract
There are compelling reasons to develop a tissue-engineered mitral valve, but this endeavor has not received the same attention as tissue engineering strategies for the semilunar valves. Challenges in regenerating a mitral valve include recapitulating the complex heterogeneity in terms of anatomy (differently sized leaflets, numerous chordae), extracellular matrix composition, biomechanical behavior, valvular interstitial cell and endothelial cell phenotypes, and interior vasculature and innervation. It will also be essential to restore the functional relationships between the native mitral valve and left ventricle. A growing amount of information relevant to tissue engineering a mitral valve has been recently collected through investigations of cell mechanobiology and collagen organization. It is hoped that the development of tissue-engineered mitral valves can build on knowledge derived from engineering semilunar valves, but the mitral valve will present its own unique challenges as investigators move toward a first-generation prototype.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lloyd-Jones D, Adams RJ, Brown TM, et al.: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121:e46–e215.
Fedak PW, McCarthy PM, Bonow RO: Evolving concepts and technologies in mitral valve repair. Circulation 2008, 117:963–974.
Kunzelman KS, Cochran RP, Murphree SS, et al.: Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J Heart Valve Dis 1993, 2:236–244.
Stephens EH, Chu CK, Grande-Allen KJ: Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. Acta Biomater 2008, 4:1148–1160.
Grashow JS, Yoganathan AP, Sacks MS: Biaixal stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann Biomed Eng 2006, 34:315–325.
May-Newman K, Yin FC: Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am J Physiol 1995, 269:H1319–1327.
• Aldous IG, Veres SP, Jahangir A, Lee JM: Differences in collagen cross-linking between the four valves of the bovine heart: A possible role in adaptation to mechanical fatigue. Am J Physiol Heart Circ Physiol 2009, 296:H1898–1906. This work finds considerable differences in collagen cross-linking between the semilunar and atrioventricular valves, which may have implications for the fundamental distinctions between aortic valve disease (calcific) and MV disease (myxomatous).
Liao J, Vesely I: A structural basis for the size-related mechanical properties of mitral valve chordae tendineae. J Biomech 2003, 36:1125–1133.
Liao J, Priddy LB, Wang B, et al.: Ultrastructure of porcine mitral valve chordae tendineae. J Heart Valve Dis 2009, 18:292–299.
•• Swanson JC, Davis LR, Arata K, et al.: Characterization of mitral valve anterior leaflet perfusion patterns. J Heart Valve Dis 2009, 18:488–495. This study is the first in vivo demonstration of perfusion of the blood vessels within the MV leaflets and chordae.
Ritchie J, Warnock JN, Yoganathan AP: Structural characterization of the chordae tendineae in native porcine mitral valves. Ann Thorac Surg 2005, 80:189–197.
Duran CM, Gunning AJ: The vascularization of the heart valves: A comparative study. Cardiovasc Res 1968, 2:290–296.
Borin C, Vanhercke D, Weyns A: Innervation of the atrioventricular and semi-lunar heart valves: A review. Acta Cardiol 2006, 61:463–469.
Kunzelman KS, Cochran RP: Stress/strain characteristics of porcine mitral valve tissue: Parallel versus perpendicular collagen orientation. J Card Surg 1992, 7:71–78.
Liao J, Yang L, Grashow J, Sacks MS: The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng 2007, 129:78–87.
Stephens EH, de Jonge N, McNeill MP, et al.: Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng Part A 2010, 16:867–878.
Chen L, McCulloch AD, May-Newman K: Nonhomogeneous deformation in the anterior leaflet of the mitral valve. Ann Biomed Eng 2004, 32:1599–1606.
Sacks MS, Enomoto Y, Graybill JR, et al.: In-vivo dynamic deformation of the mitral valve anterior leaflet. Ann Thorac Surg 2006, 82:1369–1377.
Jensen AS, Baandrup U, Hasenkam JM, et al.: Distribution of the microelastic properties within the human anterior mitral leaflet. Ultrasound Med Biol 2006, 32:1943–1948.
Grashow JS, Sacks MS, Liao J, Yoganathan AP: Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Ann Biomed Eng 2006, 34:1509–1518.
Obadia JF, Casali C, Chassignolle JF, Janier M: Mitral subvalvular apparatus: Different functions of primary and secondary chordae. Circulation 1997, 96:3124–3128.
Lomholt M, Nielsen SL, Hansen SB, et al.: Differential tension between secondary and primary mitral chordae in an acute in-vivo porcine model. J Heart Valve Dis 2002, 11:337–345.
Lim KO, Boughner DR: Morphology and relationship to extensibility curves of human mitral valve chordae tendineae. Circ Res 1976, 39:580–585.
Liao J, Vesely I: Skewness angle of interfibrillar proteoglycans increases with applied load on mitral valve chordae tendineae. J Biomech 2007, 40:390–398.
Padala M, Sacks MS, Liou SW, et al.: Mechanics of the mitral valve strut chordae insertion region. J Biomech Eng 2010, 132:081004.
Liu AC, Joag VR, Gotlieb AI: The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007, 171:1407–1418.
Stephens EH, Grande-Allen KJ: Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis 2007, 16:672–682.
Stephens EH, Durst CA, Swanson JC, et al.: Functional coupling of valvular interstitial cells and collagen in the mitral leaflet. Cell Mol Bioeng 2010, 3:428–437.
Merryman WD, Youn I, Lukoff HD, et al.: Correlation between heart valve interstitial cell stiffness and transvalvular pressure: Implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol 2006, 290:H224–231.
Rabkin E, Aikawa M, Stone JR, et al.: Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001, 104:2525–2532.
Taylor PM, Batten P, Brand NJ, et al.: The cardiac valve interstitial cell. Int J Biochem Cell Biol 2003, 35:113–118.
Blevins TL, Peterson SB, Lee EL, et al.: Mitral valvular interstitial cells demonstrate regional, adhesional, and synthetic heterogeneity. Cells Tissues Organs 2008, 187:113–122.
Walker GA, Masters KS, Shah DN, et al.: Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 2004, 95:253–260.
Liu AC, Gotlieb AI: Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol 2008, 173:1275–1285.
Gotlieb AI, Rosenthal A, Kazemian P: Fibroblast growth factor 2 regulation of mitral valve interstitial cell repair in vitro. J Thorac Cardiovasc Surg 2002, 124:591–597.
Cushing MC, Mariner PD, Liao JT, et al.: Fibroblast growth factor represses smad-mediated myofibroblast activation in aortic valvular interstitial cells. Faseb J 2008, 22:1769–1777.
Stephens EH, Timek TA, Daughters GT, et al.: Significant changes in mitral valve leaflet matrix composition and turnover with tachycardia-induced cardiomyopathy. Circulation 2009, 120:S112–119.
Gupta V, Tseng H, Lawrence BD, Grande-Allen KJ: Effect of cyclic mechanical strain on glycosaminoglycan and proteoglycan synthesis by heart valve cells. Acta Biomater 2009, 5:531–540.
Gupta V, Werdenberg JA, Blevins TL, Grande-Allen KJ: Synthesis of glycosaminoglycans in differently loaded regions of collagen gels seeded with valvular interstitial cells. Tissue Eng 2007, 13:41–49.
Gupta V, Werdenberg JA, Lawrence BD, et al.: Reversible secretion of glycosaminoglycans and proteoglycans by cyclically stretched valvular cells in 3d culture. Ann Biomed Eng 2008, 36:1092–1103.
• Dal-Bianco JP, Aikawa E, Bischoff J, et al.: Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation 2009, 120:334–342. This investigation builds on previous studies of MV remodeling in functional mitral regurgitation by demonstrating that the valvular cells undergo a reactivation of embryonic remodeling pathways.
Flanagan TC, Black A, O’Brien M, et al.: Reference models for mitral valve tissue engineering based on valve cell phenotype and extracellular matrix analysis. Cells Tissues Organs 2006, 183:12–23.
Flanagan TC, Wilkins B, Black A, et al.: A collagen-glycosaminoglycan co-culture model for heart valve tissue engineering applications. Biomaterials 2006, 27:2233–2246.
Simmons CA, Grant GR, Manduchi E, Davies PF: Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res 2005, 96:792–799.
Shi Y, Vesely I: Characterization of statically loaded tissue-engineered mitral valve chordae tendineae. J Biomed Mater Res A 2004, 69:26–39.
Vesely I, Shi Y, Dobkin D, et al.: Progress in developing a composite tissue-engineered aortic valve. Conf Proc IEEE Eng Med Biol Soc 2005, 7:7129–7130.
Shi Y, Rittman L, Vesely I: Novel geometries for tissue-engineered tendonous collagen constructs. Tissue Eng 2006, 12:2601–2609.
Gheewala N, Grande-Allen KJ: Design and mechanical evaluation of a physiological mitral valve organ culture system. Cardiovasc Eng Technol 2010, 1:123–131.
•• Lieber SC, Kruithof BP, Aubry N, et al.: Design of a miniature tissue culture system to culture mouse heart valves. Ann Biomed Eng 2010, 38:674–682. This new bioreactor device will allow the in vitro culture of a mouse heart. Using this system, scientists can leverage the enormous range of knockout and transgenic mouse models of cardiovascular and other diseases to understand the impact of these genes on the biology and pathology of heart valves and other cardiac tissues.
•• Chiu YN, Norris RA, Mahler G, et al.: Transforming growth factor beta, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: Relevance for heart valve tissue engineering. Tissue Eng Part A 2010, 16:3375–3383. This work exemplifies the innovative combination of developmental biology and bioengineering strategies to understand the remodeling principles that are desired in the regeneration of replacement tissues. These approaches can also be used to investigate pathologic remodeling of heart valves, because it is believed that some valve disease mechanisms may invoke regression to embryonic phenotypes.
Stephens EH, Durst CA, West JL, Grande-Allen KJ: Mitral valvular interstitial cell responses to substrate stiffness depend on age and anatomic region. Acta Biomater 2011, 7:75–82.
Acknowledgment
K.J. Grande-Allen has received grant support from the National Institutes of Health and the National Science Foundation. J. Liao has received grant support from the NIH.
Disclosure
Conflicts of interest: K.J. Grande-Allen: has received honoraria from the Methodist Hospital CME program; and has received honoraria and travel expenses from the Georgia Tech/Hilton Head Conference on Valve Biology and Tissue Engineering; J. Liao: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grande-Allen, K.J., Liao, J. The Heterogeneous Biomechanics and Mechanobiology of the Mitral Valve: Implications for Tissue Engineering. Curr Cardiol Rep 13, 113–120 (2011). https://doi.org/10.1007/s11886-010-0161-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-010-0161-2