Abstract
Purpose of Review
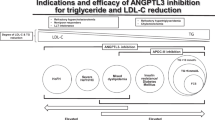
The role of the inhibition of ANGPTL3 in severe or refractory hypercholesterolemia is well documented, less in severe hyperTG. This review focuses on the preclinical and clinical development of ApoC-III inhibitors and ANGPTL3, 4, and 3/8 complex inhibitors for the treatment of severe or refractory forms of hypertriglyceridemia to prevent cardiovascular disease or other morbidities.
Recent Findings
APOC3 and ANGPTL3 became targets for drug development following the identification of naturally occurring loss of function variants in families with a favorable lipid profile and low cardiovascular risk. The inhibition of ANGPTL3 covers a broad spectrum of lipid disorders from severe hypercholesterolemia to severe hypertriglyceridemia, while the inhibition of ApoC-III can treat hypertriglyceridemia regardless of the severity.
Summary
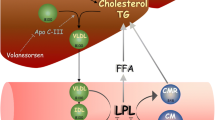
Preclinical and clinical data suggest that ApoC-III inhibitors, ANGPTL3 inhibitors, and inhibitors of the ANGPTL3/8 complex that is formed postprandially are highly effective for the treatment of severe or refractory hypertriglyceridemia. Inhibition of ANGPTL3 or the ANGPTL3/8 complex upregulates LPL and facilitates the hydrolysis and clearance of triglyceride-rich lipoproteins (TRL) (LPL-dependent mechanisms), whereas ApoC-III inhibitors contribute to the management and clearance of TRL through both LPL-dependent and LPL-independent mechanisms making it possible to successfully lower TG in subjects completely lacking LPL (familial chylomicronemia syndrome). Most of these agents are biologicals including monoclonal antibodies (mAb), antisense nucleotides (ASO), small interfering RNA (siRNA), or CRISPR-cas gene editing strategies.


Similar content being viewed by others
Abbreviations
- ANGPTL:
-
Angiopoietin-like protein
- Apo:
-
Apolipoprotein
- ASO:
-
Antisense oligonucleotide
- CVD:
-
Cardiovascular disease
- FCS:
-
Familial chylomicronemia syndrome
- GalNAc:
-
N-acetylgalactosamine
- GPIHBP1:
-
Glycosylphosphatidylinositol anchored high-density lipoprotein binding protein 1
- HDL-C:
-
High-density lipoprotein-cholesterol
- HoFH:
-
Homozygous familial hypercholesterolemia
- HyperTG:
-
Hypertriglyceridemia
- LDL-C:
-
Low-density lipoprotein-cholesterol
- LDLR:
-
Low-density lipoprotein receptor
- LMF1:
-
Lipase maturation factor 1
- LoF:
-
Loss-of-function
- LPL:
-
Lipoprotein lipase
- mAb:
-
Monoclonal antibody
- MCT:
-
Medium chain triglyceride
- mRNA:
-
Messenger RNA
- NAFLD:
-
Non-alcoholic fatty liver disease
- PLC:
-
Platelet count
- siRNA:
-
Small interfering RNA
- SRB1:
-
Scavenger receptor class B member 1
- TG:
-
Triglyceride
- TRL:
-
Triglyceride-rich lipoprotein
- VLDL:
-
Very low-density lipoprotein
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42(47):4791–806. This expert consensus paper highlights the importance of TRL in cardiovascular risk management and facilitates the classification of hyperTG.
Brunzell JD, Deeb SS. Familial lipoprotein lipase deficiency, Apo C-II deficiency, and hepatic lipase deficiency. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA, editors. The Online Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill Education; 2019.
Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol. 2016;27(3):233–41.
Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64(23):2525–40.
Gaudet D. 35 - Special Patient Populations: Treatment of Familial Chylomicronemia Syndrome and Sustained Chylomicronemia. In: Ballantyne CM, editor. Clinical Lipidology (Third Edition). New Delhi: Elsevier; 2024. p. 336–44.e2. In this textbook in clinical lipidology, recent data on hyperTG vs cardiovascular risk and emerging therapies are presented (chap 4, 27, 28, and 35 specifically.
Brisson D, Larouche M, Chebli J, Khoury E, Gaudet D. Correlation between chylomicronemia diagnosis scores and post-heparin lipoprotein lipase activity. Clin Biochem. 2023;114:67–72.
Moulin P, Dufour R, Averna M, Arca M, Cefalu AB, Noto D, et al. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): Expert panel recommendations and proposal of an “FCS score.” Atherosclerosis. 2018;275:265–72. This is the first clinical diagnosis scoring system presented to distinguish between FCS and multifactorial chylomicronemia.
Berberich AJ, Hegele RA. A modern approach to dyslipidemia. Endocr Rev. 2022;43(4):611–53.
De Villers-Lacasse A, PaqueAe M, Baass A, Bernard S. Non-alcoholic faAy liver disease in patients with chylomicronemia syndromes. J Clin Lipidol. 2023;17(4):475–82.
Paquette M, Bernard S. The evolving story of multifactorial chylomicronemia syndrome. Front Cardiovasc Med. 2022;9:886266.
Brahm AJ, Hegele RA. Chylomicronaemia—current diagnosis and future therapies. Nat Rev Endocrinol. 2015;11(6):352–62.
Williams L, Rhodes KS, Karmally W, Welstead LA, Alexander L, Sutton L, et al. Familial chylomicronemia syndrome: bringing to life dietary recommendations throughout the life span. J Clin Lipidol. 2018;12(4):908–19.
Wu SA, Kersten S, Qi L. Lipoprotein lipase and its regulators: an unfolding story. Trends Endocrinol Metab. 2021;32(1):48–61.
Dallinga-Thie GM, Kroon J, Boren J, Chapman MJ. Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr Cardiol Rep. 2016;18(7):67.
Sylvers-Davie KL, Davies BSJ. Regulation of lipoprotein metabolism by ANGPTL3, ANGPTL4, and ANGPTL8. Am J Physiol Endocrinol Metab. 2021;321(4):E493–508.
Conklin D, Gilbertson D, Taft DW, Maurer MF, Whitmore TE, Smith DL, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62(3):477–82.
Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30(2):151–7.
Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest. 2009;119(1):70–9.
Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363(23):2220–7.
Martin-Campos JM, Roig R, Mayoral C, Martinez S, Marti G, Arroyo JA, et al. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin Chim Acta. 2012;413(5–6):552–5.
Minicocci I, Montali A, Robciuc MR, Quagliarini F, Censi V, Labbadia G, et al. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J Clin Endocrinol Metab. 2012;97(7):E1266–75.
Gusarova V, Alexa CA, Wang Y, Rafique A, Kim JH, Buckler D, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res. 2015;56(7):1308–17.
Wang Y, Gusarova V, Banfi S, Gromada J, Cohen JC, Hobbs HH. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J Lipid Res. 2015;56(7):1296–307.
Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N Engl J Med. 2017;377(3):222–32.
Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211–21.
Xu YX, Redon V, Yu H, Querbes W, Pirruccello J, Liebow A, et al. Role of angiopoietin-like 3 (ANGPTL3) in regulating plasma level of low-density lipoprotein cholesterol. Atherosclerosis. 2018;268:196–206.
Wang J, Zheng W, Zheng S, Yuan Y, Wen W, Cui W, et al. Targeting ANGPTL3 by GalNAc-conjugated siRNA ANGsiR10 lowers blood lipids with long-lasting and potent efficacy in mice and monkeys. Mol Ther Nucleic Acids. 2023;31:68–77.
Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation. 2018;137(9):975–7.
Qiu M, Glass Z, Chen J, Haas M, Jin X, Zhao X, et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc Natl Acad Sci U S A. 2021;118(10):e2020401118.
Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res. 2020;61(9):1271–86.
Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun. 2012;424(4):786–92.
Quagliarini F, Wang Y, Kozlitina J, Grishin NV, Hyde R, Boerwinkle E, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci U S A. 2012;109(48):19751–6.
Haller JF, Mintah IJ, Shihanian LM, Stevis P, Buckler D, Alexa-Braun CA, et al. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res. 2017;58(6):1166–73.
Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM, Cohen JC, et al. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc Natl Acad Sci U S A. 2013;110(40):16109–14.
Izumi R, Kusakabe T, Noguchi M, Iwakura H, Tanaka T, Miyazawa T, et al. CRISPR/Cas9-mediated Angptl8 knockout suppresses plasma triglyceride concentrations and adiposity in rats. J Lipid Res. 2018;59(9):1575–85.
Balasubramaniam D, Schroeder O, Russell AM, Fitchett JR, Austin AK, Beyer TP, et al. An anti-ANGPTL3/8 antibody decreases circulating triglycerides by binding to a LPL-inhibitory leucine zipper-like motif. J Lipid Res. 2022;63(5):100198.
Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007;39(4):513–6.
Talmud PJ, Smart M, Presswood E, Cooper JA, Nicaud V, Drenos F, et al. ANGPTL4 E40K and T266M: effects on plasma triglyceride and HDL levels, postprandial responses, and CHD risk. Arterioscler Thromb Vasc Biol. 2008;28(12):2319–25.
Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, et al. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N Engl J Med. 2016;374(12):1123–33.
Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A. 2007;104(28):11766–71.
Deng M, Kutrolli E, Sadewasser A, Michel S, Joibari MM, Jaschinski F, et al. ANGPTL4 silencing via antisense oligonucleotides reduces plasma triglycerides and glucose in mice without causing lymphadenopathy. J Lipid Res. 2022;63(7):100237.
Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, et al. Evinacumab for Homozygous Familial Hypercholesterolemia. N Engl J Med. 2020;383(8):711–20.
Banerjee P, Chan KC, Tarabocchia M, Benito-Vicente A, Alves AC, Uribe KB, et al. Functional analysis of LDLR (low-density lipoprotein receptor) variants in patient lymphocytes to assess the effect of Evinacumab in homozygous familial hypercholesterolemia patients with a spectrum of LDLR activity. Arterioscler Thromb Vasc Biol. 2019;39(11):2248–60.
Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ESG, Ali S, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383(24):2307–19.
Ahmad Z, Pordy R, Rader DJ, Gaudet D, Ali S, Gonzaga-Jauregui C, et al. Inhibition of angiopoietin-like protein 3 with Evinacumab in subjects with high and severe hypertriglyceridemia. J Am Coll Cardiol. 2021;78(2):193–5.
Ahmad Z, Banerjee P, Hamon S, Chan K-C, Bouzelmat A, Sasiela WJ, et al. Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertriglyceridemia. Circulation. 2019;140(6):470–86.
Rosenson RS, Gaudet D, Ballantyne CM, Baum SJ, Bergeron J, Kershaw EE, et al. Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial. Nat Med. 2023;29(3):729–37. This publication demonstrates clearly that patients completely lacking LPL (FCS) do not respond to ANGPTL3 inhibition treatment.
Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41(40):3936–45.
Bergmark BA, Marston NA, Bramson CR, Curto M, Ramos V, Jevne A, et al. Effect of Vupanorsen on non-high-density lipoprotein cholesterol levels in statin-treated patients with elevated cholesterol: TRANSLATE-TIMI 70. Circulation. 2022;145(18):1377–86.
Burks KH, Basu D, Goldberg IJ, Stitziel NO. Angiopoietin-like 3: An important protein in regulating lipoprotein levels. Best Pract Res Clin Endocrinol Metab. 2023;37(3):101688.
WaAs GF, Schwabe C, ScoA R, Gladding PA, Sullivan D, Baker J, et al. RNA interference targeting ANGPTL3 for triglyceride and cholesterol lowering: phase 1 basket trial cohorts. Nat Med. 2023;29(9):2216–23.
Gaudet D, Gonciarz M, Shen X, Mullins G, Leohr JK, Benichou O, et al. A first-in-human single ascending dose study of a monoclonal antibody against the ANGPTL3/8 complex in subjects with mixed hyperlipidemia. Atherosclerosis. 2022;355:12.
Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113(5):691–700.
Norata GD, Tsimikas S, Pirillo A, Catapano AL. Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol Sci. 2015;36(10):675–87.
Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol. 2012;23(3):206–12.
Taskinen MR, Packard CJ, Boren J. Emerging evidence that ApoC-III inhibitors provide novel options to reduce the residual CVD. Curr Atheroscler Rep. 2019;21(8):27.
Tg, Hdl Working Group of the Exome Sequencing Project NHL, Blood I, Crosby J, Peloso GM, Auer PL, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31.
Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322(5908):1702–5.
Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res. 2011;52(2):189–206.
Maeda N, Li H, Lee D, Oliver P, Quarfordt SH, Osada J. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J Biol Chem. 1994;269(38):23610–6.
Graham MJ, Lee RG, Bell TA 3rd, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112(11):1479–90.
Wong SC, Li Z, Given B, Seefeld M, Andersen A, Zhu R, et al. Personalized medicine for dyslipidemias by RNA interference-mediated reductions in apolipoprotein C3 or angiopoietin-like protein 3. J Clin Lipidol. 2019;13(3):e15.
Butler AA, Price CA, Graham JL, Stanhope KL, King S, Hung YH, et al. Fructose-induced hypertriglyceridemia in rhesus macaques is attenuated with fish oil or ApoC3 RNA interference. J Lipid Res. 2019;60(4):805–18.
Alexander V, Gaudet D, Cheng W, Flaim J, Hughes S, Singleton W, et al. An antisense inhibitor of apolipoprotein C-III significantly decreases apolipoprotein C-III, triglycerides, very-low-density lipoprotein cholesterol and particle number, and increases high-density lipoprotein cholesterol and particle number in hypertriglyceridemic patients on a fibrate. J Am Coll Cardiol. 2014;63(12_Supplement):A1453-A.
Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371(23):2200–6.
Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373(5):438–47.
Oral EA, Garg A, Tami J, Huang EA, O’Dea LSL, Schmidt H, et al. Assessment of efficacy and safety of volanesorsen for treatment of metabolic complications in patients with familial partial lipodystrophy: Results of the BROADEN study: Volanesorsen in FPLD; The BROADEN Study. J Clin Lipidol. 2022;16(6):833–49.
Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9(5):264–75. Results of this study highlighted that ApoC-III inhibition importantly decreases TG levels in a large spectrum of hyperTG phenotypes.
Larouche M, Brisson D, Morissette MC, Gaudet D. Post-prandial analysis of fluctuations in the platelet count and platelet function in patients with the familial chylomicronemia syndrome. Orphanet J Rare Dis. 2023;18(1):167.
Gaudet D, Clifton P, Sullivan D, Baker J, Schwabe C, Thackwray S, et al. RNA interference therapy targeting apolipoprotein C-III in hypertriglyceridemia. NEJM Evid. 2023;0(0):EVIDoa2200325.
Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Prevalence of US Adults with Triglycerides >/= 150 mg/dl: NHANES 2007–2014. Cardiol Ther. 2020;9(1):207–13.
Ruiz-Garcia A, Arranz-Martinez E, Lopez-Uriarte B, Rivera-Teijido M, Palacios-Martinez D, Davila-Blazquez GM, et al. Prevalence of hypertriglyceridemia in adults and related cardiometabolic factors. SIMETAP-HTG study. Clin Investig Arterioscler. 2020;32(6):242–55.
Larouche M, Pordy R, Banerjee P, Gaudet D. Clinical trial with the ANGPTL3 monoclonal antibody evinacumab identifies a new rare chylomicronemia causing variant in the LPL gene. Canadian Journal of Cardiology. 2023;39(10):S183.
Author information
Authors and Affiliations
Contributions
All co-authors have participated to the writing and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
DG Employer: Université de Montréal; Consultation fees: Amryt (Chiesi), Arrowhead, CRISPR Therapeutics, Eli Lilly, Ionis, Pfizer, Regeneron; Trust research/joint research funds (trials): Arrowhead, Eli Lilly, Ionis, Kowa, Pfizer, Regeneron. ML, EK, and DB have nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Larouche, M., Khoury, E., Brisson, D. et al. Inhibition of Angiopoietin-Like Protein 3 or 3/8 Complex and ApoC-III in Severe Hypertriglyceridemia. Curr Atheroscler Rep 25, 1101–1111 (2023). https://doi.org/10.1007/s11883-023-01179-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01179-y