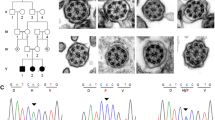
Abstract
Otitis media with effusion (OME) is the most common cause of conductive hearing loss in children and is strongly associated with primary ciliary dyskinesia (PCD). Approximately half of the children with PCD require otolaryngology care, posing a major problem in this population. Early diagnosis of PCD is critical in these patients to minimise the collateral damage related to OME. The current gold standard for PCD diagnosis requires determining ciliary structure defects by transmission electron microscopy (TEM) or clearly documenting ciliary dysfunction via digital high-speed video microscopy (DHSV). Although both techniques are useful for PCD diagnosis, they have limitations and need to be supported by new methodologies, including genetic analysis of genes related to PCD. In this article, we review classical and recently associated mutations related to ciliary alterations leading to PCD, which can be useful for early diagnosis of the disease and subsequent early management of OME.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–9.
Rossman CM, Forrest JB, Lee RM, Newhouse MT. The dyskinetic cilia syndrome: ciliary motility in immotile cilia syndrome. Chest. 1980;78:580–2.
Afzelius BA, Mossberg B. Immotile-cilia syndrome (primary ciliary dyskinesia), including Kartagener syndrome. In: Scriver C, Beaudet AL, Sly W, Valle D, editors. The metabolic and molecular bases of inherited diseases. 7th ed. New York: McGraw-Hill; 1995. p. 3943–54.
Afzelius BA. Situs inversus and ciliary abnormalities. What is the connection? Int J DevBiol. 1995;39:839–44.
Afzelius BA. The immotile-cilia syndrome: a microtubule-associated defect. CRC Crit Rev Biochem. 1985;19:63–87.
Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. EurRespir J. 1997;10:2376–9.
Lous J, Burton MJ, Felding FU, Ovesen T, Rovers MM, Williamson I. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801.
Simpson SA, Thomas CL, van der Linden MC, Macmillan H, van der Wouden JC, Butler C. Identification of children in the first four years of life for early treatment for otitis media with effusion. Cochrane Database Syst Rev. 2007;1:CD004163.
Wolter NE, Dell SD, James AL, Campisi P. Middle ear ventilation in children with primary ciliary dyskinesia. Int J Pediatr Otorhinolaryngol. 2012;76:1565–8.
Majithia A, Fong J, Hariri M, Harcourt J. Hearing outcomes in children with primary ciliary dyskinesia–a longitudinal study. Int J Pediatr Otorhinolaryngol. 2005;69:1061–4.
Casselbrant ML, Mandel EM, Jung J, Ferrell RE, Tekely K, Szatkiewicz JP, et al. Otitis media: a genome-wide linkage scan with evidence of susceptibility loci within the 17q12 and 10q22.3 regions. BMC Med Genet. 2009;10:85.
Bush A, Cole P, Hariri M, Mackay I, Phillips G, O’Callaghan C, et al. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J. 1998;12:982–8.
Armengot M, Bonet M, Carda C, Gómez MJ, Milara J, Mata M, et al. Development and validation of a method of cilia motility analysis for the early diagnosis of primary ciliary dyskinesia. Acta Otorrinolaringol Esp. 2012;63:1–8.
Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–67.
Carda C, Armengot M, Escribano A, Peydro A. Ultrastructural patterns of primary ciliar dyskinesia syndrome. Ultrastruct Pathol. 2005;29:3–8.
Jorissen M, Willems T, Van der SB Verbeken E, De Boeck K. Ultrastructural expression of primary ciliary dyskinesia after ciliogenesis in culture. Acta Otorhinolaryngol Belg. 2000;54:343–56.
Escudier E, Couprie M, Duriez B, Roudot- Thoraval F, Millepied MC, Pruliere-Escabasse V, et al. Computer-assisted analysis helps detect inner dynein arm abnormalities. Am J Respir Crit Care Med. 2002;166:1257–62.
Mata M, Sarrion I, Armengot M, Carda C, Martinez I, Melero JA, et al. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine. PLoS One. 2012;7:e48037.
Mata M, Martinez I, Melero JA, Tenor H, Cortijo J. Roflumilast inhibits respiratory syncytial virus infection in human differentiated bronchial epithelial cells. PLoS One. 2013;8:e69670.
Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473–87.
Olbrich H, Haffner K, Kispert A, Volkel A, Volz A, Sasmaz G, et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet. 2002;30:143–4.
Loges NT, Olbrich H, Fenske L, Mussaffi H, Horvath J, Fliegauf M, et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am J Hum Genet. 2008;83:547–58.
Pennarun G, Escudier E, Chapelin C, Bridoux AM, Cacheux V, Roger G, et al. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet. 1999;65:1508–19.
Mazor M, Alkrinawi S, Chalifa-Caspi V, Manor E, Sheffield VC, Aviram M, et al. Primary ciliary dyskinesia caused by homozygous mutation in DNAL1, encoding dynein light chain 1. Am J Hum Genet. 2011;88:599–607. This study demonstrates that a homozygous point mutation in DNAL1 is associated with absent or markedly shortened ODAs..
Duriez B, Duquesnoy P, Escudier E, Bridoux AM, Escalier D, Rayet I, et al. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2007;104:3336–41.
Mitchison HM, Schmidts M, Loges NT, Freshour J, Dritsoula A, Hirst RA, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet. 2012;44(381–9):S1–2.
Omran H, Kobayashi D, Olbrich H, Tsukahara T, Loges NT, Hagiwara H, et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456:611–6.
Loges NT, Olbrich H, Becker-Heck A, Häffner K, Heer A, Reinhard C, et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am J Hum Genet. 2009;85:883–9.
Panizzi JR, Becker-Heck A, Castleman VH, Al-Mutairi DA, Liu Y, Loges NT, et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat Genet. 2012;44:714–9.
Kott E, Duquesnoy P, Copin B, Legendre M, Dastot-Le Moal F, Montantin G, et al. Loss-of-function mutations in LRRC6, a gene essential for proper axonemal assembly of inner and outer dynein arms, cause primary ciliary dyskinesia. Am J Hum Genet. 2012;91:958–64.
Castleman VH, Romio L, Chodhari R, Hirst RA, de Castro SC, Parker KA, et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet. 2009;84:197–209.
Merveille AC, Davis EE, Becker-Heck A, Legendre M, Amirav I, Bataille G, et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet. 2011;43:72–8.
Becker-Heck A, Zohn IE, Okabe N, Pollock A, Lenhart KB, Sullivan-Brown J, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet. 2011;43:79–84. The authors identify an uncharacterized coiled-coil domain containing a protein, CCDC40, essential for correct left-right patterning.
Olbrich H, Schmidts M, Werner C, Onoufriadis A, Loges NT, Raidt J, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet. 2012;91:672–84. Using high screening SNPs methodologies, the authors find a PCD-associated mutation in HYDIN causing a premature protein termination in respiratory cells and not affecting nodal cilia function.
Haimo LT, Rosenbaum JL. Cilia, flagella, and microtubules. J Cell Biol. 1981;91:125–30.
Chodhari R, Mitchison HM, Meeks M. Cilia, primary ciliary dyskinesia and molecular genetics. Paediatr Respir Rev. 2004;5:69–76.
Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–10.
Wagner MK, Yost HJ. Left-right development: the roles of nodal cilia. Curr Biol. 2000;10:R149–51.
Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37.
Pigino G, Ishikawa T. Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture. 2012;2:50–8.
Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61.
Satir P. Structural basis of ciliary movement. Environ Health Perspect. 1980;35:77–82.
Kamiya R. Exploring the function of inner and outer dynein arms with Chlamydomonas mutants. Cell Motil Cytoskeleton. 1995;32:98–102.
Smith EF, Lefebvre PA. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil Cytoskeleton. 1997;38:1–8.
Dutcher SK, Huang B, Luck DJ. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J Cell Biol. 1984;98:229–36.
Brokaw CJ, Luck DJ, Huang B. Analysis of the movement of Chlamydomonas flagella: the function of the radial-spoke system is revealed by comparison of wild-type and mutant flagella. J Cell Biol. 1982;92:722–32.
Afzelius B. Electron microscopy of the sperm tail; results obtained with a new fixative. J Biophys Biochem Cytol. 1959;5:269–78.
Pigino G, Bui KH, Maheshwari A, Lupetti P, Diener D, Ishikawa T. Cryoelectron tomography of radial spokes in cilia and flagella. J Cell Biol. 2011;195:673–87.
Satir P. Studies on cilia. 3. Further studies on the cilium tip and a “sliding filament” model of ciliary motility. J Cell Biol. 1968;39:77–94.
Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–33.
Wright CV. Mechanisms of left-right asymmetry: what's right and what's left? Dev Cell. 2001;1:179–86.
Zhou H, Wang X, Brighton L, Hazucha M, Jaspers I, Carson JL. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009;21:875–81.
Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23:175–81.
Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827–35.
Dirksen ER, Sanderson MJ. Regulation of ciliary activity in the mammalian respiratory tract. Biorheology. 1990;27:533–45.
Sanderson MJ, Dirksen ER. Mechanosensitive and beta-adrenergic control of the ciliary beat frequency of mammalian respiratory tract cells in culture. Am Rev Respir Dis. 1989;139:432–40.
Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N, et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2002;99:10282–6.
Schwabe GC, Hoffmann K, Loges NT, Birker D, Rossier C, de Santi MM, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29:289–98.
Guichard C, Harricane MC, Lafitte JJ, Godard P, Zaegel M, Tack V, et al. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome). Am J Hum Genet. 2001;68:1030–5.
Zariwala M, Noone PG, Sannuti A, Minnix S, Zhou Z, Leigh MW, et al. Germline mutations in an intermediate chain dynein cause primary ciliary dyskinesia. Am J Respir Cell Mol Biol. 2001;25:577–83.
Zariwala MA, Leigh MW, Ceppa F, Kennedy MP, Noone PG, Carson JL, et al. Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation. Am J Respir Crit Care Med. 2006;174:858–66.
Escudier E, Duquesnoy P, Papon JF, Amselem S. Ciliary defects and genetics of primary ciliary dyskinesia. Paediatr Respir Rev. 2009;10:51–4.
Geremek M, Zietkiewicz E, Diehl SR, Alizadeh BZ, Wijmenga C, Witt M. Linkage analysis localises a Kartagener syndrome gene to a 3.5 cM region on chromosome 15q24-25. J Med Genet. 2006;43:e1.
Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013;93:711–20.
Knowles MR, Leigh MW, Carson JL, Davis SD, Dell SD, Ferkol TW, et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012;67:433–41. The authors demonstrate that mutations in DNAH11 are common causes of PCD patients without ciliary ultrastructural defects..
Pifferi M, Michelucci A, Conidi ME, Cangiotti AM, Simi P, Macchia P, et al. New DNAH11 mutations in primary ciliary dyskinesia with normal axonemal ultrastructure. Eur Respir J. 2010;35:1413–6.
Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ, et al. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013;92:99–106. This article emphasises the association of CCDC114 with PCD and the usefulness of exome sequencing to identify genetic causes in heterogeneous recessive disorders..
Onoufriadis A, Paff T, Antony D, Shoemark A, Micha D, Kuyt B, et al. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am J Hum Genet. 2013;92:88–98. The study demonstrates that deficiency of CCDC114 causes a complete absence of ciliary ODAs.
Takada S, Wilkerson CG, Wakabayashi K, Kamiya R, Witman GB. The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol Biol Cell. 2002;13:1015–29.
Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–21.
Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–76.
Freshour J, Yokoyama R, Mitchell DR. Chlamydomonas flagellar outer row dynein assembly protein ODA7 interacts with both outer row and I1 inner row dyneins. J Biol Chem. 2007;282:5404–12.
Duquesnoy P, Escudier E, Vincensini L, Freshour J, Bridoux AM, Coste A, et al. Loss-of-function mutations in the human ortholog of Chlamydomonas reinhardtii ODA7 disrupt dynein arm assembly and cause primary ciliary dyskinesia. Am J Hum Genet. 2009;85:890–6.
Taipale M, Kaminen N, Nopola-Hemmi J, Haltia T, Myllyluoma B, Lyytinen H, et al. A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proc Natl Acad Sci U S A. 2003;100:11553–8.
Tarkar A, Loges NT, Slagle CE, Francis R, Dougherty GW, Tamayo JV, et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat Genet. 2013;45:995–1003. This study proposes DYX1C1 as a new dynein axonemal assembly factor DNAAF2..
Moore DJ, Onoufriadis A, Shoemark A, Simpson MA, Zur Lage PI, de Castro SC, et al. Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am J Hum Genet. 2013;93:346–56. The authors conclude that ZMYND10 is required for IDA and ODA assembly and that alterations of this protein cause PCD with laterality defects.
Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–50.
Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA, et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation. 2012;125:2232–42.
Blanchon S, Legendre M, Copin B, Duquesnoy P, Montantin G, Kott E, et al. Delineation of CCDC39/CCDC40 mutation spectrum and associated phenotypes in primary ciliary dyskinesia. J Med Genet. 2012;49:410–6. The authors propose CCDC39 and CCDC40 mutation as the major cause of PCD with IDA defects, which can be useful for the diagnosis of this alteration, often difficult to be discriminated by electron microscopy.
Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, Forouhan M, et al. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat. 2013;34:462–72.
Narayan D, Krishnan SN, Upender M, Ravikumar TS, Mahoney MJ, Dolan Jr TF, et al. Unusual inheritance of primary ciliary dyskinesia (Kartagener's syndrome). J Med Genet. 1994;31:493–6.
van Dorp DB, Wright AF, Carothers AD, Bleeker-Wagemakers EM. A family with RP3 type of X-linked retinitis pigmentosa: an association with ciliary abnormalities. Hum Genet. 1992;88:331–4.
Iannaccone A, Breuer DK, Wang XF, Kuo SF, Normando EM, Filippova E, et al. Clinical and immunohistochemical evidence for an X linked retinitis pigmentosa syndrome with recurrent infections and hearing loss in association with an RPGR mutation. J Med Genet. 2003;40:e118.
Moore A, Escudier E, Roger G, Tamalet A, Pelosse B, Marlin S, et al. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006;43:326–33.
Budny B, Chen W, Omran H, Fliegauf M, Tzschach A, Wisniewska M, et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet. 2006;120(2):171–8.
Krawczyński MR, Witt M. PCD and RP: X-linked inheritance of both disorders? Pediatr Pulmonol. 2004;38:88–9.
Zito I, Downes SM, Patel RJ, Cheetham ME, Ebenezer ND, Jenkins SA, et al. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J Med Genet. 2003;40:609–15.
Hong DH, Pawlyk B, Sokolov M, Strissel KJ, Yang J, Tulloch B, et al. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–21.
Schermer B, Höpker K, Omran H, Ghenoiu C, Fliegauf M, Fekete A, et al. Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia. EMBO J. 2005;24:4415–24.
Bukowy-Bieryłło Z, Ziętkiewicz E, Loges NT, Wittmer M, Geremek M, Olbrich H, et al. RPGR mutations might cause reduced orientation of respiratory cilia. Pediatr Pulmonol. 2013;48:352–63. This study confirms that RPGR mutations affect the ciliary beat coordination and the proper respiratory cilia orientation, contributing to the PCD phenotype.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Manuel Mata, Lara Milian, Miguel Armengot and Carmen Carda declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Otitis
Rights and permissions
About this article
Cite this article
Mata, M., Milian, L., Armengot, M. et al. Gene Mutations in Primary Ciliary Dyskinesia Related to Otitis Media. Curr Allergy Asthma Rep 14, 420 (2014). https://doi.org/10.1007/s11882-014-0420-1
Published:
DOI: https://doi.org/10.1007/s11882-014-0420-1