Abstract
The fine particulate matter samples for 24 h were carried out at the Environment Monitoring Station (EMS) and Shandong Jianzhu University (SJU) sites during 2010 in Jinan City, China. Eight water-soluble ion species were analyzed by ion chromatography, while organic carbon (OC) and elemental carbon (EC) were determined with the IMPROVE thermal optical reflectance method, and 20 inorganic elements were measured by inductively coupled plasma-atomic emission spectrometer and inductively coupled plasma-mass spectroscopy. The annual average mass concentration of PM2.5 was 168.85 μg m−3 at EMS and 148.67 μg m−3 at SJU. The coefficient of divergence was 0.14, 0.19, 0.23, and 0.23 in spring, summer, fall, and winter, respectively, indicating that there was no obvious spatial difference at the two sampling sites. The highest PM2.5, OC, and OC/EC ratio were in winter because of the enhanced emissions from coal combustion for heating and poor atmospheric dispersion. By the method of enrichment factors, the 20 inorganic elements were divided into three types owing to their sources. Al, Si, and Ti were mainly contributed by crustal sources. Na, Mg, P, K, Ca, V, Cr, Mn, Fe, Co, Ni, Ba, and Sr were from both natural emissions and anthropogenic sources. Cu, Zn, Pb, and Sn mainly originated from anthropogenic sources such as vehicular exhaust and industrial emission. Chemical mass closure calculation estimated that SO4 2− was the largest contributor and explained 29.66 % of PM2.5 mass at EMS, while 31.64 % was at SJU. The organic matter, crustal matter, and NO3 –, respectively, accounted for 15.12, 12.87, and 13.77 % to PM2.5 at EMS, while it accounted for 13.46, 13.96, and 14.93 % at SJU, respectively. By the positive matrix factorization analysis, the coal combustion and biomass burning, secondary sulfate, soil dust, secondary nitrate, and vehicle emissions were identified as the major emission sources.
Similar content being viewed by others
Introduction
Atmospheric aerosol particles play an important role in air quality and climate system as well as the control of physical and chemical processes of the atmosphere. In recent decades, concerns had been raised and it indicated that atmospheric fine particles (PM2.5, particles with an aerodynamic diameter of 2.5 μm or less) and their poisonous components (heavy metals, PAHs, etc.) were responsible for adverse health and environmental effects (Schwartz et al. 1996; Vedal 1997; Norris et al. 1999; Menon et al. 2002; Ostro et al. 2006; Dockery and Stone 2007; Duan et al. 2007; 2013). Airborne fine particles led to smog and haze formation that reduced visibility (Watson 2002; Kim et al. 2007). Major chemical components of PM2.5 had been reported as organic and element carbon, sulfate, and nitrate (Harrison 2004; Walker et al. 2004; Reiss et al. 2007) and were the significant causes of visibility degradation (Chow et al. 1996; Chan et al. 1997; Cao et al. 2007).
In China, studies for PM2.5 had been gradually carried out since 2000 and mainly focused on the urban areas of the Pearl River Delta, Beijing, Tianjin, and Xi’an. Some studies gave the general characteristics of PM2.5 chemical compositions and discussed their seasonal variations, correlations, or sources (He et al. 2001; Yao et al. 2002, 2003; Dan et al. 2004; Sun et al. 2004; Huang et al. 2006; Song et al. 2006; Cheng et al. 2009; Wang et al. 2009; Guo et al. 2010; Ianniello et al. 2011; Gu et al. 2011; Li et al. 2012; Tao et al. 2012). However, PM2.5 had seldom been sampled and chemically analyzed in Jinan City, and related published data for PM2.5 in urban areas of Jinan are also short. To better control the local aerosol pollution and implement the New National Ambient Air Quality Standard promulgated on February 2012, obtaining the information of local PM2.5 mass concentration, chemical composition, pollution source, and spatial and temporal variation is quite necessary.
Jinan is the capital of Shandong Province and one of the important city agglomerations in China. The total amount of population had exceeded 6.0 million inhabitants on a 153.5-km2 area in 2010. Although the structures of fuel consumption have always been changing as result of the rapid economic development, coal is still the primary fuel in Jinan and widely used for industrial processes and daily life, especially more coal is combusted for heating between November and March due to the cold winter. The amount of motor vehicles now exceeds 1.3 million and is annually growing at a rate of nearly 19 %. The Jinan City experiences a continental monsoon climate, with hot, humid summer and dry, cold winter. The mean annual precipitation is 619 mm, and nearly half of the annual rainfall occurs in July and August. Rapid urbanization and economic development are deteriorating Jinan’s environmental air quality. Previous works about atmospheric fine particles in Jinan tended to the particle size fractions (Gao et al. 2007) and limited to chemical compositions. The main objectives of this study are to (1) characterize mass concentration variation for PM2.5 and major chemical compositions; (2) determine the relative contribution of chemical compositions by the method of mass closure; (3) discriminate the spatial difference for PM2.5 and its major chemical compositions; and (4) estimate the source contribution by using positive matrix factorization (PMF).
Materials and methods
Sampling sites
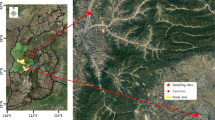
The locations of the two sampling sites were shown in Fig. 1.
EMS
The sampling location is on the top floor of Jinan Environment Monitoring Station (117°2′55″E, 36°39′44″N), about 15 m high above ground level in downtown area. It is close to residential buildings, so the pollutant from cooking emission will not be ignored. In addition, the concentration of contaminants in gaseous and condensed phase can be assumed to be strongly affected by the traffic due to the two major roadways of the district. The sampling site represents a mixed residential/commercial/industrial area.
SJU
The sampling site is located on the rooftop of the science and technology building in Shandong Jianzhu University (117°11′9″E, 36°40′53″N). It is approximately 15 m above ground and surrounded by three major roadways. But the traffic volume of these roadways is not extremely high compared with that of Environment Monitoring Station (EMS). The sampling site is experiencing a rapid economic development and abundant construction activities. The sampling site represents a newly formed urban area.
Sample collection
Atmospheric particle sampling was respectively performed in 2010. The 24-h PM2.5 samples were synchronously collected on 90-mm quartz fibers and 90-mm polypropylene fiber filters at the two sampling sites using medium-volume samplers (model TH-150S, manufactured by Tianhong Instrument Co., Ltd. Wuhan, China) operating at a flow rate of 100 L min−1 with a 2.5-μm cutpoint impactor. To remove volatile components that might be present on the filter before sampling, the quartz fiber filters were preheated in the muffle furnace at 600 °C for 3 h, while the polypropylene fiber filters were in the oven at 60 °C for 1 h. Before and after sampling, the filters were equilibrated in a dessicator (temperature 20–23 °C and relative humidity (RH) 35–45 %) for 48 h and then weighed on an electronic microbalance with a ±1-μg sensitivity (Mettler Toledo Inc., Switzerland). After that, the samples were stored in a refrigerator at −18 °C before chemical analysis.
Eliminating the invalid atmospheric particle samples due to the rain, sampler’s malfunction, filter’s fracture, or other unexpected accidents, the available PM2.5 quartz fiber and polypropylene fiber filter samples were synchronously collected, 18, 11, 17, and 14 in spring, summer, fall, and winter, respectively, at EMS and Shandong Jianzhu University (SJU) in 2010.
Chemical analysis
Organic carbon (OC) and elemental carbon (EC) were measured by the IMPROVE thermal optical reflectance method with Desert Research Institute Model 2001 Thermal Optical Carbon Analyzer (Chow et al. 2001, 2004, 2005). Details of the analytical procedure as well as QA/QC were given by Cao et al. (2005).
Ion chromatography (ICS-1500, Dionex Ltd., USA) was used to analyze the three anions (Cl–, NO3 –, and SO4 2–) and five cations (Na+, NH4 +, K+, Mg2+, and Ca2+). Principal and details of the analytical procedure were given by Gu et al. (2011).
Inductively coupled plasma-atomic emission spectrometer (Baldwin et al. 1994) (IRIS Intrepid II, Thermo Electron) and inductively coupled plasma-mass spectroscopy (Chio et al. 2004; Kong et al. 2011) (Agilent 7500a, Agilent Co. USA) analyses were employed for the determination of Na, Mg, Al, Si, P, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Pb, Ba, Sr, and Sn in samples collected on polypropylene fiber filters.
Other data collection
The data of SO2 and NO2 were all obtained from Jinan Environment Monitoring Station. The corresponding meteorological data (temperature, wind speed, RH, etc.) were obtained from Jinan Meteorological Bureau.
Results and discussion
PM2.5 mass concentration
Statistics for particulate mass concentration at the two sampling sites were shown in Table 1 during the observation period. The annual arithmetic mean of PM2.5 was 168.85 μg m−3 at EMS and 147.33 μg m−3 at SJU, respectively. The lowest mass concentrations were 65.45 and 44.10 μg m−3, which occurred on April 23 and April 29, while the highest concentrations were 563.16 μg and 559.13 μg m−3, which occurred on January 29 and January 19 at EMS and SJU, respectively. It was noted that the minimum mass concentration was all presented at low wind speed (1.5 m/s) and after rain in spring, while the maximum concentration was also presented at low wind speed (1.7 m/s) but before rain or snow in winter at both sampling sites. It indicated that the meteorological conditions were important to particulate convergence and diffusion in Jinan.
The diurnal mass concentrations of PM2.5 were compared to the Ambient Air Quality Standards (GB3095-2012) of class II (75 μg m−3) promulgated by the Ministry of Environmental Protection of the People’s Republic of China for PM2.5. It was found that there were 23 PM2.5 samples at EMS and 19 PM2.5 samples at SJU, whose concentration exceeded the standard of GB3095-2012; furthermore, the PM2.5 mass concentration level was the standard by 1.4–9.1 times during the sampling period in Jinan, indicating that the PM2.5 pollution was quite serious and could not be ignorable in the future.
From the seasonal variation of PM2.5, the highest PM2.5 mass concentration was in winter at both sampling sites. When many heating systems were run by burning coal, particulate emission increased during the winter. Moreover, in winter, the weather in Jinan was usually influenced by high pressure systems, which caused formation of inversion layers that could inhibit pollutant dispersion. In the present study, the average wind speed was 3.75, 2.90, 2.92, and 2.99 m/s, RH 46.6, 68.4, 56.4, and 44.6 %, and temperature 13.7, 26.4, 15.6, and 1.5 °C in spring, summer, fall, and winter, respectively. In spring, the weather was windy and dry and favorable for dispersion of PM, at the same time the low relative humidity might not favor to secondary particle formation. In summer, the rainfall was very plentiful and the PM could be efficiently removed by wet scavenging. But dust events and high RH frequently occurred in spring and summer, respectively, so the PM2.5 mass concentration did not have obvious seasonal fluctuation in spring and summer. It should be noted that the PM2.5 mass concentration was higher at SJU than that at EMS only in fall. Agrarian cultivation in SJU suburbs was still extensive. Both the burning crop remnants and fertilizing the soil were contributed to the higher PM2.5 mass concentration. Except in fall, the arithmetic means of PM2.5 in other seasons were all higher at EMS than that at SJU, which might have the high traffic flows and the emissions from the nearby industrial and restaurants cooking at EMS. Figure 2 showed the spatial variation of PM2.5 mass concentration during the observation period. When the PM2.5 mass concentration was lower than 100 μg m−3, there was no obvious difference in PM2.5 mass concentration at two sampling sites. However, the spatial difference was more obvious with the increase of the diurnal PM2.5 mass concentration, especially when the mass concentration was larger than 200 μg m−3.
Coefficient of divergence (CD) (Wongphatarakul et al. 1998; Zhang and Friedlander 2000; Park and Kim 2004) was used to test the extent of spatial difference. The CD was defined as follows:
where j and k represented two sampling sites, and p was the number of chemical components. x ij was the average mass concentration for a chemical component i at site j. The CD approached zero, indicating the little difference between them, and approached one, indicating the big difference.
In order to analyze the spatial difference of PM2.5 and chemical species at the two sampling sites, the CDs of PM2.5 and chemical species were calculated and the values were 0.14 in spring, 0.19 in summer, 0.23 in fall, and 0.23 in winter, respectively. These CDs were little and adjacent zero, indicating that there were no obvious difference for the PM2.5 and chemical species at the two sampling sites. So, the regional pollution character of the fine particles was present in Jinan, China.
Chemical compositions
Carbonaceous components
The overall average mass concentration of the OC and EC components in PM2.5 was 17.69 and 5.53 μg m−3 at EMS, while it was 14.29 and 5.12 μg m−3 at SJU, respectively. The carbonaceous pollution exhibited obvious seasonal characteristics. Table 1 presented the order of OC seasonal variation: winter > autumn > spring > summer at both sampling sites. This seasonal variation could be attributed to the cooperative effects of changes in emission rate and meteorological conditions. In winter, the highest concentration of OC could be attributed to the enhanced emission from coal combustion heating and unfavorable atmospheric dispersion (low mixing layer height, frequent inversion, etc.). The smallest concentration of OC was in summer for the decreasing consumption of coal, and more rainy days aided the dispersion and mitigated the carbonaceous pollution. The seasonal variation of EC at EMS site was autumn > winter > spring > summer, while at the SJU site, the variation was autumn > winter > summer > spring, respectively. These variations were distinct from what was observed for OC. The differences of seasonal variation between OC and EC suggested that emission sources for OC and EC were probably different.
At EMS, the proportion of OC for PM2.5 was 9.75, 8.77, 13.56, and 12.45 % in spring, summer, fall, and winter, while it was 10.17, 7.21, 11.28, and 12.10 % at SJU, respectively. The proportions of EC for PM2.5 were all less than 5.00 % in all the seasons at both sampling sites. It could indicate that coal combustion heating and biomass fuels led to more carbonaceous species enriched in fine particles. Because the relative abundances of OC and EC, respectively, determined the relative amounts of scattering and absorption, the OC abundance was higher than EC abundance in all the seasons at two sampling sites in this study, indicating that the light scattering of carbonaceous aerosol should be the more important factor causing visibility degradation in Jinan.
The ratio of OC to EC (OC/EC) can be used to interpret the emission and transformation characteristics of carbonaceous aerosol. As shown in Table 1, the diurnal ratio of OC/EC was the highest in winter at both sampling sites. This seasonal pattern of higher wintertime OC/EC ratio was also observed in Beijing (Dan et al. 2004), Guangzhou and Hong Kong (Duan et al. 2007), and Tianjin (Gu et al. 2011). The EC tracer method was used to calculate the seasonal mass concentration of secondary organic carbon (SOC) (Turpin and Huntzicker 1995; Castro et al. 1999; Cao et al. 2004, 2005, 2007). The seasonal SOC mass concentration and the ratio of SOC/OC were listed in Table 2 using the EC tracer method in this study. Similar to OC, SOC was also highest in winter and lowest in summer. This might be attributed to several reasons. First, coal consumption for winter heating contributed more to OC than EC and also increased the emission of volatile organic precursors. Second, low temperature led to the adsorption and condensation of semi-volatile organic compounds onto existing solid particles. Third, the low mixing layer height in winter would enhance the SOC formation.
Inorganic water-soluble ions
Figure 3 presents the mass concentration of major water-soluble ions (Na+, NH4 +, K+, Mg2+, Ca2+, Cl−, NO3 −, SO4 2−) in PM2.5. The average concentration of SO4 2− was higher in summer, fall, and winter than that in spring at both sampling sites. Although the concentration of SO2 was relatively lower than that in winter, the sulfur oxidation ratio SOR ([SO4 2–]/([SO2] + [SO4 2–])) was higher than 0.1 in primary pollutants (Wang et al. 2005) for the strong photochemical reaction. Higher SOR suggested the oxidation of gaseous species and more secondary aerosols in the atmosphere. In this study, the SOR was 0.22, 0.55, 0.34, and 0.16 in spring, summer, fall, and winter at EMS, while it was 0.25, 0.55, 0.37, and 0.14 at SJU, respectively, indicating that SOR was highest in summer and lowest in winter. In summer, the gas particle conversion and further condensation or adsorption on the particle surface were likely the major sources of the secondary sulfate particles for the high temperature, and long photoperiod created the favorable photoreaction conditions for photochemical oxidation. At the same time, high relative humidity in summer could increase the heterogeneous oxidation reactions involving SO2 in clouds and fog (Yao et al. 2002; Zhou et al. 2012). Although the meteorological conditions (such as stable atmospheric boundary layer and the low humid elimination efficiency) completely led to the lower rate of the second conversion of SO2 to SO4 2−, the union effects of the sufficient SO2 and liquid-phase reaction and substantial anthropogenic sources jointly led to the relatively high concentration of SO4 2− in winter.
The highest seasonal average of NO3 − was 38.35 and 33.00 μg m−3 in winter at EMS and SJU, respectively. Compared to SO4 2−, the NO3 − had not evidently fluctuated. The nitrate had weak thermal stability and was more sensitive to temperature. Higher temperature did not favor the formation of nitrate. The nitrogen oxidation ratio (NOR = [NO3 –] / ([NO2] + [NO3 –])) could be used to assess the transformation degree of gaseous NO2 to aqueous nitrate. In this study, the NOR was 0.27, 0.20, 0.17, and 0.27 in spring, summer, fall, and winter at EMS, while it was 0.28, 0.23, 0.18, and 0.25 at SJU, respectively, indicating that the formation degree of NO3 – from NO2 was very weak. And that the annual average ratios of NO3 –/SO4 2– were 0.89 and 0.45 at EMS and SJU, respectively. The lower value of NO3 –/SO4 2– at SJU could also reflect the dominated coal combustion sources for particles. The higher value of NO3 –/SO4 2– at EMS depicted that more fractions of particles resulted from motor vehicle exhaust.
Compared to SO4 2– and NO3 –, the concentration of Cl− was relatively low in all the seasons. The ratio of Cl–/Na+ was about 1.2 in seawater (Tsitouridou and Samara 1993). But in this study, the highest ratio of Cl–/Na+ was in winter and the value was 10.07 and 9.59, much larger than 1.2, at EMS and SJU, respectively, indicating that most of the chloride might not be present as sea salt particles. Possibly, it originated from industrial contamination sources or, else, the chloride which originated from large sea salt particles, but was displaced by reaction with nitric acid and followed by reaction with ammonia to form small particles (Wang and Hu 2000).
Agricultural activities had been considered to be the major emission source for NH3 in atmosphere (Sutton et al. 1995). Ammonium ion was usually transformed from ammonia in atmosphere. In the present study, the highest concentration of NH4 + was observed in summer, likely because of the increasing of the high temperature, increased animal activities, and more frequent agricultural activities. The mass concentration of Ca2+ was relatively higher in spring and winter and was related to dry weather, low precipitation, construction activities, and strong winds, which resulted in both local and long-distance transport dust events. The seasonal variation of other three cations (Na+, K+, and Mg2+) was very little.
Inorganic elements
Average mass concentrations of 20 inorganic elements at EMS and SJU were listed in Table 2. At both sampling sites, the sum of element mass concentration was higher in winter and spring than that in fall and summer. Moreover, of these elements, Al, Si, K, Ca, and Fe exhibited especially high concentration, indicating that the mineral dust pollution for fine particles was very serious. Table 2 also presented the obvious seasonal variation for elements. The highest mass concentration appearing in spring at SJU was likely ascribed to the soil, non-mineral dust, or dust storm contribution. But at EMS, the highest concentrations of Si, Al, Ca, and Fe appeared in winter, indicating that there were no significant contributions of soil and non-mineral dust; probably, this was due to in part to fly ash. Additional influences from cement dust and other construction-related activities would also appear to be possible contributors owing to the rapid urbanization. For the other trace elements, their concentrations were similar and there was no obvious variation in spring, summer, and fall, while the anthropogenic sources contributed to the maximum concentration in winter.
In order to evaluate the contributions of crustal and non-crustal sources, one way to estimate the possible source of the trace elements associated with the PM2.5 could be carried out by calculating the enrichment factors (EF). For each element, the EF was calculated according to the equation given below:
Where X was the trace element of interest, Y was the reference element, while (X/Y)sample and (X/Y)crust were the ratio between the concentration of the trace element of interest in the air and geological material, respectively. In this study, Al was used as the reference element for abundance in the upper continental crust. The EF was approximate to the unit, indicating that the soil and geological materials were the dominant sources for PM2.5. The EF was respectively 1–2, 2–5, 5–20, and 20–40, not only indicating that both natural emissions and anthropogenic sources were important for these elements, but also indicating that the enrichment degrees were slight, medium, notable, and intensity, respectively. The EF was larger than 40, indicating that the enrichment degree was pole strength. In this study, Al was selected as the reference element because it was relatively stable and was not affected by most anthropogenic contaminants. The denary logarithm of EFs was shown in Fig. 4. At both sampling sites, the EF values of Si and Ti were approximate to the unit, indicating that they were mainly contributed by crustal source. The EF values of Na, Mg, P, K, Ca, V, Cr, Mn, Fe, Co, Ni, Ba, and Sr were mainly between 1 and 40, suggesting that both natural emissions and anthropogenic sources were important for these elements. The EF values of Cu, Zn, Pb, and Sn were larger than 40, indicating that they mainly originated from non-crustal sources such as vehicular exhaust and industrial emission.
Mass closure analysis
The method of mass closure for particles had become the accepted method for the analysis of aerosols (Chan et al. 1997; Christoforou et al. 2000). Normally, PM2.5 could be classified into six major types: organic matter, major water soluble ions, element carbon, crustal matter, sea salt, and trace species. Since the chemical composition of organic fraction for aerosol was largely unknown. Organic matter was obtained by multiplying OC by a factor of 1.4, as was commonly used to estimate the unmeasured hydrogen and oxygen in organic compounds. Soluble Na in the aerosol samples was assumed to come solely from sea salt; thus, the mass of sea salt was estimated by 2.54 × Na+. The mass of crustal matter was estimated on the basis of the oxides of Al, Ca, Si, Ti, Fe, Na, Mg, and K as follows:
The sum of all other elements was defined as trace species, including P, V, Cr, Mn, Co, Ni, Ba, Sr, Cu, Zn, Pb, and Sn in this study.
The average contribution percentages of the major types for PM2.5 were given in Fig. 5. The sum of contribution percentage for the nine chemical components was approximately 86.77 and 89.25 % of the PM2.5 mass concentration at EMS and SJU, respectively. At the both sampling sites, the SO4 2−, NO3 −, organic carbon, and crustal matter were the main contribution, and the sums of their percentage abundance were 71.44 and 73.99 % at EMS and SJU, respectively. This indicated that it was very pivotal to control the four chemical components in order to reduce the pollution of PM2.5 in Jinan. The higher percentage abundance of organic carbon should be at EMS than that at SJU. The percentage abundance of crustal matter was similar to that in Beijing (Yang et al. 2004) and higher than that in Tianjin (Gu et al. 2011). Maybe it indicated that there were many construction activities and special geographical situation to the disadvantage of diffusion. The unidentified average portion was 13.23 and 10.25 % of the PM2.5 at EMS and SJU, respectively. Their components probably included unmeasured crustal matter, trace elements, and moisture absorbed by particles or might have been due to systematic weighing errors.
Source apportionment by PMF
PMF (v3.0, USEPA, 2008) was described in detail in Pattero and Tapper (1994) and Pattero (2004) and used to identify the contribution of various emission sources. As suggested by several recent source apportionment studies (Lee et al. 1999; Liu et al. 2005; Song et al. 2006; Raman and Hopke 2007), the mass fraction distribution of species was used to identify the sources, which included soil dust, vehicle emissions, sea salt, industrial emissions, and secondary aerosols.
By the PMF analysis, the source profiles were displayed in Fig. 6. The source enriched in NH4 + and SO4 2− was classified as secondary sulfate in the previous studies (Kim et al. 2007; Raman and Hopke 2007). The secondary sulfates formed by photochemical and other chemical processes were known to be major constituents of fine particulate matter measured in most industrialized areas. In this study, NH4 + and SO4 2− were composed of higher mass fractions and indicated secondary sulfate source. Soil dust, including airborne soil and road dust, included most of the crustal elements and had high concentrations of Al, Ca, Fe, Si, Na, Mg, and K. Coal combustion and biomass burning were presented by high OC and EC (Zheng et al. 2005; Watson 2002). Coal is still a major energy source in Jinan. Moreover, agrarian cultivation in Jinan suburbs is still extensive. Secondary nitrate is characterized by high nitrate. The precursor gas of nitrate, NO x , is emitted by traffic and stationary source such as electricity-generating plants. Motor vehicle emissions are characterized by high Pb, Sn, and EC. The source concentrations to PM2.5 were as follows: coal combustion and biomass burning (38.00 %), secondary sulfate (34.98 %), secondary nitrate and vehicle emissions (16.85 %), and soil dust (10.17 %) at EMS and coal combustion and biomass burning (46.23 %), soil dust (16.91 %), and secondary sulfate (36.86 %) at SJU.
Conclusions
A 1-year-long observation of PM2.5 in Jinan was carried out in 2010. The PM2.5 mass concentration and major chemical components were synchronously investigated at two sampling sites. The annual mean mass concentration of PM2.5 was 168.85 μg m−3 at EMS and 148.67 μg m−3 at SJU, respectively. The PM2.5 mass concentration with obvious seasonal variation character resulted from varying emission sources and meteorological conditions. The coefficients of dispersion were calculated in each season and the values were all adjacent zero, showing that there was no obvious spatial difference and character of regional air pollution in Jinan. The highest carbon concentration and OC/EC ratios were in winter and resulted from enhanced emissions from coal combustion for heating and poor atmospheric dispersion. On the contrary, the abundant rainfall removed carbonaceous aerosol by wet scavenging and led to the lowest carbon concentration and OC/EC ratio in summer. SO4 2−, NO3 −, and NH4 + were the main chemical components of PM2.5 in Jinan. SO4 2− and NH4 + concentrations were highest in summer, while NO3 − was highest in autumn. The seasonal concentration variation for different ions resulted from the source variation, secondary aerosol ion formation mechanisms, and meteorological conditions. By the method of chemistry mass closure, approximately 86.77 and 89.25 % of the PM2.5 mass concentrations were explained by nine chemical components and sulfate was the most predominant fraction accounting for about 29.66 and 31.64 % of PM2.5 at EMS and SJU site, respectively. By the PMF analysis, the coal combustion and biomass burning, secondary aerosol, and soil dust were identified as the major emission sources in Jinan.
References
Baldwin AT, Schmelz EA, Ohnmeiss TE (1994) Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. J Chem Ecol 20(8):2139–2157
Cao JJ, Lee SC, Ho KF, Zou SC, Fung K, Li Y, Watson JG, Chow JC (2004) Spatial and seasonal variations of atmospheric organic carbon and elemental carbon in Pearl River Delta Region, China. Atmos Environ 38(27):4447–4456
Cao JJ, Wu F, Chow JC, Lee SC, Li Y, Chen SW, An ZS, Fung KK, Watson JG, Zhu CS, Liu SX (2005) Characterization and source apportionment of atmospheric organic and elemental carbon during fall and winter of 2003 in Xi’an, China. Atmos Chem Phys 5:3127–3137
Cao JJ, Lee SC, Chow JC, Watson JG, Ho KF, Zhang RJ, Jin ZD, Shen ZX, Chen GC, Kang YM, Zou LZ, Zhang LZ, Qi Sh, Dai MH, Cheng Y, Hu K (2007) Spatial and seasonal distributions of carbonaceous aerosols over China. J Geophys Res 112 (D22), doi:10.1029/2006JD008205.
Castro LM, Pio CA, Harrison RM, Smith DJT (1999) Carbonaceous aerosol in urban and rural European atmospheres: estimation of secondary organic carbon concentrations. Atmos Environ 33(17):2771–2781
Chan YC, Simpson RW, Mctainsh GH, Vowle PD (1997) Characterization of chemical species in PM2.5 and PM10 aerosols in Brisbane, Australia. Atmos Environ 31(22):2061–2080
Cheng YF, Berghof M, Garland RM, Wiedensohler A, Wehner B, Müller T, Su H, Zhang YH, Achter P, Nowak A, Pöschl U, Zhu T, Hu M, Meng LM (2009) Influence of soot mixing state on aerosol light absorption and single scattering albedo during air mass 15 aging at a polluted regional site in northeastern China. J Geophys Res 114(D2):D00G10, doi:10.1029/2008JD010883
Chio C, Cheng M, Wang C (2004) Source apportionment to PM in different air quality conditions for Taichung urban and coastal areas, Taiwan. Atmos Environ 38(39):6893–6905
Chow JC, Watson JG, Lu ZQ, Lowenthal DH, Frazier CA, Solomon PA, Thuillier RH, Magliano K (1996) Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmos Environ 30(12):2079–2112
Chow JC, Watson JG, Crow D, Lowenthal DH, Merrifield T (2001) Comparison of IMPROVE and NIOSH carbon measurements. Aerosol Sci Technol 34(1):23–34
Chow JC, Watson JG, Chen LWA, Arnott WP, Moosmüller H (2004) Equivalence of elemental carbon by thermal/optical reflectance and transmittance with different temperature protocols. Environ Sci Technol 38(16):4414–4422
Chow JC, Watson JG, Louie PK, Chen LW, Sin D (2005) Comparison of PM2.5 carbon measurement methods in Hong Kong, China. Environ Pollut 137(2):334–344
Christoforou CS, Salmon LG, Hannigan MP, Solomon PA, Cass GR (2000) Trends in fine particle concentration and chemical composition in southern California. J Air Waste Manag Assoc 50(1):43–53
Dan M, Zhuang GS, Li XX (2004) The characteristics of carbonaceous species and their sources in PM2.5 in Beijing. Atmos Environ 38(21):3443–3452
Dockery DW, Stone PH (2007) Cardiovascular risks from fine particulate air pollution. N Engl J Med 356:511–513
Duan JC, Tan JH, Cheng DX, Bi XH, Deng WJ, Sheng GY, Fu JM, Wong MH (2007) Sources and characteristics of carbonaceous aerosol in two largest cities in Pearl River Delta Region, China. Atmos Environ 41(14):2895–2903
Duan J, Tan J (2013) Atmospheric heavy metals and arsenic in China: situation, sources and control policies. Atmos Environ 74:93–101
Gao J, Wang J, Cheng SH, Xue LK, Hz Y, Hou LJ, Jiang YQ, Wang WX (2007) Number concentration and size distribution of submicron particles in Jinan urban area: characteristics in summer and winter. J Environ Sci 19(12):1466–1473
Gu JX, Bai ZP, Li AX, Wu LP, Xie YY, Lei WF, Dong HY, Zhag X (2011) Chemical composition of PM2.5 during winter in Tianjin, China. Particuology 9(3):215–221
Guo S, Hu M, Wang ZB, Wang ZB, Slanina J, Zhao YL (2010) Size-resolved aerosol water-soluble ionic compositions in the summer of Beijing: implication of regional f secondary ormation. Atmos Chem Phys 10:947–959
Harrison RM (2004) Key pollutants—airborne particles. Sci Total Environ 334–335:3–8
He KB, Yang FM, Ma YL, Ma YL, Zhang Q, Yao XH, Chan CK, Cadle S, Chan T, Mulawa P (2001) The characteristics of PM2.5 in Beijing, China. Atmos Environ 35(29):4959–4970
Huang XF, He LY, Hu M, Zhang YH (2006) Annual variation of particulate organic compounds in PM2.5 in the urban atmosphere of Beijing. Atmos Environ 40(14):2449–2458
Ianniello A, Spataro F, Esposito G, Allegrini I, Hu M, Zhu T (2011) Chemical characteristics of inorganic ammonium salts in PM2.5 in the atmosphere of Beijing (China). Atmos Chem Phys 11:10803–10822
Kim HS, Huh JB, Hopke PK, Holsen TM, Yi SM (2007) Characteristics of the major chemical constituents of PM2.5 and smog events in Seoul, Korea in 2003 and 2004. Atmos Environ 41(32):6762–6770
Kong SF, Lu B, Bai ZP, Zhao XY, Han B, Li ZY, Ji YQ, XuYH LY, Jiang H (2011) Potential threat of heavy metals in re-suspended dusts on building surfaces in oilfield city. Atmos Environ 45(25):4192–4204
Lee E, Chan CK, Paatero P (1999) Application of Positive matrix factorization in source apportionment of particle pollutants in HongKong. Atmos Environ 33:3201–3212
Li XR, Wang LL, Wang YS, Wen TX, Yang YJ, Zhao YN, Wang YF (2012) Chemical composition and size distribution of airborne particulate matters in Beijing during the 2008 Olympics. Atmos Environ 50:278–286
Liu W, Wang Y, Russell A, Edgerton LES (2005) Atmospheric aerosol over two urban–rural pairs in the southeastern United States: chemical composition and possible sources. Atmos Environ 39:4453–4470
Menon S, Hansen J, Nazarenko L, Luo YF (2002) Climate effects of black carbon aerosols in China and India. Science 297(5590):2250–2253
Norris G, YoungPong SN, Koenig JQ, Lrson TV, Sheppard L, Stout JW (1999) An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 107(6):489–493
Ostro B, Broadwin R, Green S, Feng WY, Lipsett M (2006) Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect 114(1):29–33
Park SS, Kim YJ (2004) PM2.5 particles and size-segregated ionic species measured during fall season in three urban sites in Korea. Atmos Environ 38(10):1459–1471
Paatero P, Tapper U (1994) Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5:111–126
Paatero, P (2004) User’s guide for positive matrix factorization programs PMF2 and PMF3, part 1: tutorial.
Raman RS, Hopke PK (2007) Source apportionment of fine particles utilizing partially speciated carbonaceous aerosol data at two rural locations in New York state. Atmos Environ 41:7923–7939
Reiss R, Anderson EL, Cross CE, Hidy DH, Clellan RM, Moolgavkar S (2007) Evidence of health impacts of sulfate- and nitrate-containing particles in ambient air. Inhal Toxicol 19(5):419–449
Schwartz J, Dockery DW, Neas LM (1996) Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc 46(10):927–939
Song Y, Zhang YH, Xie SD, Zeng LM, Zheng M, Salmon LG, Shao M, Slanina S (2006) Source apportionment of PM2.5 in Beijing by positive matrix factorization. Atmos Environ 40(8):1526–1537
Sun YL, Zhuang GS, Wang Y, Han L, Guo J, Dan M, Zhang WJ, Wang ZF, Hao ZP (2004) The air-borne particulate pollution in Beijing—concentration, composition, distribution and sources. Atmos Environ 38(35):5991–6004
Sutton MA, Place CJ, Eager M, Fowler D, Smith RI (1995) Assessment of the magnitude of ammonia emissions in the United Kingdom. Atmos Environ 29(12):1393–1411
Tao J, Cao JJ, Zhang RJ, Zhu LH, Zhang T, Shi S, Chan CY (2012) Reconstructed light extinction coefficients using chemical compositions of PM2.5 in winter in urban Guangzhou, China. Adv Atmos Sci 29(2):359–368
Turpin BJ, Huntzicker JJ (1995) Identification of secondary aerosol episodes and quantification of primary and secondary organic aerosol concentrations during SCAQS. Atmos Environ 29(23):3527–3544
Tsitouridou R, Samara C (1993) First results of acidic and alkaline constituents determination in air particulates of Thessaloniki, Greece. Atmos Environ, Part B Urban Atmos 27(3):313–319
Vedal S (1997) Critical review: ambient particles and health: lines that divide. J Air Waste Manag Assoc 47(5):551–581
Walker JT, Whitall DR, Robarge W, Paerl HW (2004) Ambient ammonia and ammonium aerosol across a region of variable ammonia emission density. Atmos Environ 38(9):1235–1246
Wang M, Hu M (2000) Sea salt source contribution to the aerosol in Qingdao seashore area. J Environ Sci-China 21(5):83–85
Wang Q, Shao M, Zhang Y, Wei Y, Hu M, Guo S (2009) Source apportionment of fine organic aerosols in Beijing. Atmos Chem Phys 9:8573–8585
Wang Y, Zhuang GS, Tang AH, Yuan H, Sun YL, Chen S, Zheng AH (2005) The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos Environ 39(21):3771–3784
Watson JG (2002) Critical review discussion. Visibility: science and regulation. J Air Waste Manag Assoc 52:628–713
Wongphatarakul V, Friedlander SK, Pinto JP (1998) A comparative study of PM2.5 ambient aerosol chemical databases. Environ Sci Technol 32(24):3926–3934
Yang FM, He KB, Ma YL, Zhnag Q (2004) Characterization of mass balance of PM2.5 chemical speciation in Beijing. Environ Chem-China 23(3):326–333
Yao XH, Chan CK, Fang M, Cadle S, Chan T, Mulawa P, He K, Ye B (2002) The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos Environ 36(26):4223–4234
Yao XH, Lau APS, Fang M, Chan CK, Hu M (2003) Size distributions and formation of ionic species in atmospheric particulate pollutants in Beijing, China: 1-inorganic ions. Atmos Environ 37(21):2991–3000
Zhang Z, Friedlander SK (2000) A comparative study of chemical databases for fine particle Chinese aerosols. Environ Sci Technol 34(22):4687–4694
Zheng M, Salmon LG, Schauer JJ, Zeng L, Kiang CS, Zhang Y, Cass GR (2005) Seasonal trends in PM2.5 source contributions in Beijing, China. Atmos Environ 39:3967–3976
Zhou JM, Zhang RJ, Cao JJ, Chow JC, Watson JG (2012) Carbonaceous and ionic components of atmospheric fine particles in Beijing and their impact on atmospheric visibility. Aerosol Air Qual Res 12:492–502
Acknowledgments
This study was projected by Tianjin Science and Technology Development Commission (11JCYBJC05200), Ministry of Environmental Protection of China (2010467007), and Jinan Science and Technology Development Commission (201101090).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, J., Du, S., Han, D. et al. Major chemical compositions, possible sources, and mass closure analysis of PM2.5 in Jinan, China. Air Qual Atmos Health 7, 251–262 (2014). https://doi.org/10.1007/s11869-013-0232-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-013-0232-9