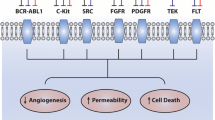
Opinion Statement
The introduction of TKIs into the therapeutic armamentarium of CML has changed the disease paradigm, increasing long-term survival from 20% to over 80%, with a life expectancy now approaching that of the general population. Although highly effective, TKIs also have a toxicity profile that is often mild to moderate, but sometimes severe, with multiple kinases involved in the development of adverse events (AEs). Among others, cardiovascular AEs observed in TKI-treated CML patients may represent a significant cause of morbidity and mortality, and their pathogenesis is still only partially understood. In view of the recent introduction into daily clinical practice of new TKIs, namely the STAMP inhibitor asciminib, with a distinct safety profile, hematologists now more than ever have the opportunity to select the most suitable TKI for each patient, an aspect that will be fundamental in terms of personalized preventive and therapeutic strategies. Furthermore, physicians should be aware of the feasibility of TKI dose modifications at all stages of the patients’ treatment journey, both at diagnosis for frail or elderly subjects or with multiple comorbidities, and during follow-up for those patients who experience toxicity, as well as to prevent it, with the main objective of reducing side effects while maintaining the response. Consequently, preserving the cardiovascular health of CML patients will likely be a more urgent topic in the near future, with specific measures aimed at controlling cardiovascular risk factors through a multidisciplinary approach involving a panel of healthcare professionals together with the hematologist.



Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Shawver LK, Slamon D, Ullrich A. Smart drugs: Tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1:117–23.
Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2018;16:1108–35.
•• Hughes TP, Mauro MJ, Cortes JE, et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N Engl J Med. 2019;381:2315–26. The first clinical trial that led to the approval of asciminib for the treatment of chronic myeloid leukemia
Wylie AA, Schoepfer J, Jahnke W, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–7.
Hantschel O, Nagar B, Guettler S, et al. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003;112:845–57.
Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
Branford S, Yeung DT, Ross DM, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121:3818–24.
Hughes T, Boquimpani C, Takahashi N, et al. Durable treatment-free remission after stopping second line nilotinib in patients with chronic myeloid leukemia in chronic phase: ENESTOP 96-wk update. Haematologica. 2017;102:75.
Ross DM, Pagani IS, Shanmuganathan N, et al. Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia. 2018;32:2572–9.
Ross DM, Hughes TP. Treatment-free remission in patients with chronic myeloid leukaemia. Nat Rev Clin Oncol. 2020;17:493–503.
•• Mahon F-X, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35. The first clinical trial to specifically evaluate the feasibility of tyrosine kinase inhibitors discontinuation in chronic myeloid leukemia patients
Campbell VE, Copland M. Dasatinib for the treatment of chronic phase chronic myeloid leukemia. Clin Pract. 2013;10:415–25.
Iurlo A, Galimberti S, Abruzzese E, et al. Pleural effusion and molecular response in dasatinib-treated chronic myeloid leukemia patients in a real-life Italian multicenter series. Ann Hematol. 2018;97:95–100.
Emir H, Albrecht-Schgoer K, Huber K, et al. Nilotinib exerts direct pro-atherogenic and antiangiogenic effects on vascular endothelial cells: A potential explanation for drug-induced vasculopathy in CML. Blood. 2013;122:257.
Hiwase DK, Carne L, Ross D, Grigg A, Hughes TP. Hypercholesterolaemia in imatinib intolerant/resistant CML-CP patients treated with nilotinib: A retrospective analysis. Blood. 2013;122:1503.
Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. J Clin Oncol. 2012;30:3486–92.
• Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: Final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404. The clinical trial that confirmed the efficacy and safety profile of ponatinib in the setting of Ph+ leukemias, including chronic myeloid leukemia
Caocci G, Mulas O, Annunziata M, et al. Long-term mortality rate for cardiovascular disease in 656 chronic myeloid leukaemia patients treated with second- and third-generation tyrosine kinase inhibitors. Int J Cardiol. 2020;301:163–6.
Manouchehri A, Kanu E, Mauro MJ, Aday AW, Lindner JR, Moslehi J. Tyrosine Kinase Inhibitors in Leukemia and Cardiovascular Events: From Mechanism to Patient Care. Arterioscler Thromb Vasc Biol. 2020;40:301–8.
Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-Associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–8.
Pandey AK, Singhi EK, Arroyo JP, et al. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor–associated hypertension and vascular disease. Hypertension. 2017;71:e1-8.
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM-L. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol. 2016;34:2851–7.
Saussele S, Krauss MP, Hehlmann R, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood. 2015;126:42–9.
Hoffmann VS, Baccarani M, Hasford J, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29:1336–43.
Aghel N, Delgado DH, Lipton JH. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance. Vasc Health Risk Manag. 2017;13:293.
Nodzon L, Fadol A, Tinsley S. Cardiovascular adverse events and mitigation strategies for chronic myeloid leukemia patients receiving tyrosine kinase inhibitor therapy. J Adv Pract Oncol. 2022;13:127–42.
Sayegh N, Yirerong J, Agarwal N, et al. Cardiovascular Toxicities Associated with Tyrosine Kinase Inhibitors. Curr Cardiol Rep. 2023;25:269–80.
Milojkovic D, Lyon AR, Mehta P, et al. Cardiovascular risk in chronic myeloid leukaemia: A multidisciplinary consensus on screening and management. Eur J Haematol. 2023;111:201–10.
Smith G, Apperley J, Milojkovic D, et al. A British Society for Haematology guideline on the diagnosis and management of chronic myeloid leukaemia. Br J Haematol. 2020;191:171–93.
•• Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. The latest version of the ELN recommendations for the diagnosis and treatment of chronic myeloid leukemia
Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv261.
Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–361.
Cohen MH, Williams G, Johnson JR, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935–42.
O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17.
Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–61.
Hochhaus A, Larson RA, Guilhot F, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376:917–27.
Atallah E, Durand J-B, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233–7.
Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29:1123–32.
Kerkelä R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16.
Wolf A, Couttet P, Dong M, et al. Imatinib does not induce cardiotoxicity at clinically relevant concentrations in preclinical studies. Leuk Res. 2010;34:1180–8.
Giles FJ, Mauro MJ, Hong F, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or nontyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27:1310–5.
Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–9.
Montani D, Bergot E, Günther S, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–37.
Fox LC, Cummins KD, Costello B, et al. The incidence and natural history of dasatinib complications in the treatment of chronic myeloid leukemia. Blood Adv. 2017;1:802–11.
Shah NP, Kantarjian HM, Kim D-W, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–12.
Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869–74.
Lilly MB, Ottmann OG, Shah NP, et al. Dasatinib 140 mg once daily versus 70 mg twice daily in patients with Ph-positive acute lymphoblastic leukemia who failed imatinib: Results from a phase 3 study. Am J Hematol. 2010;85:164–70.
Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116:3852–61.
Kantarjian H, Cortes J, Kim D-W, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113:6322–9.
Ottmann O, Saglio G, Apperley JF, et al. Long-term efficacy and safety of dasatinib in patients with chronic myeloid leukemia in accelerated phase who are resistant to or intolerant of imatinib. Blood Cancer J. 2018;8:88.
Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70.
Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34:2333–40.
Cortes JE, Jiang Q, Wang J, et al. Dasatinib vs. imatinib in patients with chronic myeloid leukemia in chronic phase (CML-CP) who have not achieved an optimal response to 3 months of imatinib therapy: the DASCERN randomized study. Leukemia. 2020;34:2064–73.
Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in Imatinib-Resistant CML and Philadelphia Chromosome-Positive ALL. N Engl J Med. 2006;354:2542–51.
Aichberger KJ, Herndlhofer S, Schernthaner GH, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86:533–9.
Le Coutre P, Rea D, Abruzzese E, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103:1347–8.
Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: A single institution study. Eur J Haematol. 2013;90:531–2.
Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–8.
Gugliotta G, Castagnetti F, Breccia M, et al. Treatment-free remission in chronic myeloid leukemia patients treated front-line with nilotinib: 10-year follow-up of the GIMEMA CML 0307 study. Haematologica. 2022;107:2356–64.
Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–7.
Masarova L, Cortes JE, Patel KP, et al. Long-Term Results of a Phase 2 Trial of Nilotinib 400 mg Twice Daily in Newly Diagnosed Patients With Chronic-Phase Chronic Myeloid Leukemia. Cancer. 2020;126:1448–59.
Saglio G, Kim D-W, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9.
Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–203.
Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Kantarjian HM, Hughes TP, Larson RA, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53.
Cortes JE, Jean Khoury H, Kantarjian H, et al. Long-term evaluation of cardiac and vascular toxicity in patients with Philadelphia chromosome-positive leukemias treated with bosutinib. Am J Hematol. 2016;91:606–16.
Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–76.
Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298–307.
Brümmendorf TH, Cortes JE, de Souza CA, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015;168:69–81.
Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety of bosutinib versus imatinib in the phase 3 BELA trial in newly diagnosed chronic phase chronic myeloid leukemia. Am J Hematol. 2014;89:947–53.
Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial. J Clin Oncol. 2018;36:231–7.
Brümmendorf TH, Cortes JE, Milojkovic D, et al. Bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: final results from the BFORE trial. Leukemia. 2022;36:1825–33.
Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96.
Lipton JH, Chuah C, Guerci-Bresler A, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:612–21.
Dorer DJ, Knickerbocker RK, Baccarani M, et al. Impact of dose intensity of ponatinib on selected adverse events: Multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 2016;48:84–91.
Cortes J, Apperley J, Lomaia E, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138:2042–50.
Iurlo A, Cattaneo D, Malato A, et al. Low-dose ponatinib is a good option in chronic myeloid leukemia patients intolerant to previous TKIs. Am J Hematol. 2020;95:E260–3.
Schoepfer J, Jahnke W, Berellini G, et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J Med Chem. 2018;61:8120–35.
Mauro MJ, Hughes TP, Kim D-W, et al. Asciminib monotherapy in patients with CML-CP without BCR::ABL1 T315I mutations treated with at least two prior TKIs: 4-year phase 1 safety and efficacy results. Leukemia. 2023;37:1048–59.
Réa D, Mauro MJ, Boquimpani C, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138:2031–41.
Hochhaus A, Réa D, Boquimpani C, et al. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. 2023;37:617–26.
Breccia M, Piciocchi A, Abruzzese E, et al. Italian Physicians’ Perceptions about the Role of Asciminib in Later Lines Chronic Myeloid Leukemia in Clinical Practice: A GIMEMA Survey. J Clin Med. 2023;12:5267.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–337.
Tini G, Sarocchi M, Tocci G, et al. Arterial hypertension in cancer: The elephant in the room. Int J Cardiol. 2019;281:133–9.
Romanens M, Sudano I, Adams A, Schober EA. Sonographic assessment of carotid atherosclerosis: preferred risk indicator for future cardiovascular events? Swiss Med Wkly. 2019;149:w20142.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27:593–603.
Preiss D, Tobert JA, Hovingh GK, Reith C. Lipid-Modifying Agents, From Statins to PCSK9 Inhibitors: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:1945–55.
Caocci G, Mulas O, Capodanno I, et al. Low low-density lipoprotein (LDL), cholesterol and triglycerides plasma levels are associated with reduced risk of arterial occlusive events in chronic myeloid leukemia patients treated with ponatinib in the real-life. A Campus CML study. Blood Cancer J. 2020;10:66.
Caocci G, Mulas O, Capodanno I, et al. Low-density lipoprotein (LDL) levels and risk of arterial occlusive events in chronic myeloid leukemia patients treated with nilotinib. Ann Hematol. 2021;100:2005–14.
Iurlo A, Orsi E, Cattaneo D, et al. Effects of first- and second-generation tyrosine kinase inhibitor therapy on glucose and lipid metabolism in chronic myeloid leukemia patients: a real clinical problem? Oncotarget. 2015;6:33944–51.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104.
Mulas O, Caocci G, Stagno F, et al. Renin angiotensin system inhibitors reduce the incidence of arterial thrombotic events in patients with hypertension and chronic myeloid leukemia treated with second- or third-generation tyrosine kinase inhibitors. Ann Hematol. 2020;99:1525–30.
Breccia M, Pregno P, Spallarossa P, et al. Identification, prevention and management of cardiovascular risk in chronic myeloid leukaemia patients candidate to ponatinib: an expert opinion. Ann Hematol. 2017;96:549–58.
Marquis-Gravel G, Roe MT, Harrington RA, Muñoz D, Hernandez AF, Jones WS. Revisiting the Role of Aspirin for the Primary Prevention of Cardiovascular Disease. Circulation. 2019;140:1115–24.
Caocci G, Mulas O, Annunziata M, et al. Cardiovascular toxicity in patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors in the real-life practice: Identification of risk factors and the role of prophylaxis. Am J Hematol. 2018;93:E159–61.
Caocci G, Mulas O, Bonifacio M, et al. Recurrent arterial occlusive events in patients with chronic myeloid leukemia treated with second- and third-generation tyrosine kinase inhibitors and role of secondary prevention. Int J Cardiol. 2019;288:124–7.
Caocci G, Mulas O, Abruzzese E, et al. Arterial occlusive events in chronic myeloid leukemia patients treated with ponatinib in the real-life practice are predicted by the Systematic Coronary Risk Evaluation (SCORE) chart. Hematol Oncol. 2019;37:296–302.
Iurlo A, Cattaneo D, Bucelli C, Breccia M. Dose Optimization of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: A New Therapeutic Challenge. J Clin Med. 2021;10:515.
Author information
Authors and Affiliations
Contributions
All authors wrote the paper and gave their final approval.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iurlo, A., Cattaneo, D., Bucelli, C. et al. Cardiovascular Adverse Events of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: Clinical Relevance, Impact on Outcome, Preventive Measures and Treatment Strategies. Curr. Treat. Options in Oncol. 24, 1720–1738 (2023). https://doi.org/10.1007/s11864-023-01149-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01149-1