Opinion statement
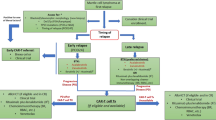
There is no standard approach to sequencing novel therapies in mantle cell lymphoma (MCL). For initial treatment, intensive induction chemotherapy followed by autologous stem cell transplant and rituximab maintenance remains our preferred approach in young, fit patients. We consider bendamustine plus rituximab or lenalidomide plus rituximab in patients who are ineligible for intensive chemotherapy-based approaches. Bruton’s tyrosine kinase inhibitors are our preferred class of agents to use in the second-line setting. When patients inevitably relapse on one of these agents, we proceed with chimeric antigen receptor T-cell (CAR T) therapy in eligible patients, often with the use of bridging therapy with corticosteroids, lenalidomide, or venetoclax. We treat patients who are ineligible for CAR T or clinic trial with venetoclax, lenalidomide, or proteosome inhibitor-based regimens, although efficacy is expected to be limited in this setting with a shortened duration of response to each subsequent line of therapy. Allogeneic stem cell transplant remains an option for carefully selected patients who progress after autologous stem cell transplant and CAR T. Clinical trials involving combinations of novel agents in early lines of therapy are ongoing, and new compounds with unique mechanisms of action are in development. The results of ongoing clinical trials with novel agents will further change the treatment landscape for patients with MCL in the coming years.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. https://doi.org/10.1182/blood-2016-01-643569.
Epperla N, Hamadani M, Fenske TS, Costa LJ. Incidence and survival trends in mantle cell lymphoma. Br J Haematol. 2018;181(5):703–6. https://doi.org/10.1111/bjh.14699.
Martin P, Chadburn A, Christos P, Weil K, Furman RR, Ruan J, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–13. https://doi.org/10.1200/jco.2008.19.6121.
Cohen JB, Han X, Jemal A, Ward EM, Flowers CR. Deferred therapy is associated with improved overall survival in patients with newly diagnosed mantle cell lymphoma. Cancer. 2016;122(15):2356–63. https://doi.org/10.1002/cncr.30068.
Kumar A, Ying Z, Alperovich A, Dogan A, Hamlin PA Jr, Moskowitz CH, et al. Outcome of initial observation in mantle cell lymphoma. Blood. 2016;128(22):1803. https://doi.org/10.1182/blood.V128.22.1803.1803.
Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–65. https://doi.org/10.1182/blood-2007-06-095331.
Eskelund CW, Dahl C, Hansen JW, Westman M, Kolstad A, Pedersen LB, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903–10. https://doi.org/10.1182/blood-2017-04-779736.
Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13):1250–60. https://doi.org/10.1056/NEJMoa1701769.
Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Geisler CH, Trneny M, et al. Treatment of older patients with mantle cell lymphoma (MCL): long-term follow-up of the randomized European MCL elderly trial. J Clin Oncol. 2020;38(3):248–56. https://doi.org/10.1200/jco.19.01294.
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–10. https://doi.org/10.1016/s0140-6736(12)61763-2.
Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–52. https://doi.org/10.1182/blood-2013-11-531327.
Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944–53. https://doi.org/10.1056/NEJMoa1412096.
Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med. 2015;373(19):1835–44. https://doi.org/10.1056/NEJMoa1505237.
Kumar A, Sha F, Toure A, Dogan A, Ni A, Batlevi CL, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9(6):50. https://doi.org/10.1038/s41408-019-0209-5.
Wiestner A. Targeting B-cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31(1):128–30. https://doi.org/10.1200/jco.2012.44.4281.
Kenkre VP, Kahl BS. The future of B-cell lymphoma therapy: the B-cell receptor and its downstream pathways. Curr Hematol Malig Rep. 2012;7(3):216–20. https://doi.org/10.1007/s11899-012-0127-0.
Merolle MI, Ahmed M, Nomie K, Wang ML. The B cell receptor signaling pathway in mantle cell lymphoma. Oncotarget. 2018;9(38):25332–41. https://doi.org/10.18632/oncotarget.25011.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–16. https://doi.org/10.1056/NEJMoa1306220.
Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739–45. https://doi.org/10.1182/blood-2015-03-635326.
Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–8. https://doi.org/10.1016/s0140-6736(15)00667-4.
Wang M, Goy A, Martin P, Ramchandren R, Alexeeva J, Popat R, et al. Efficacy and safety of single-agent ibrutinib in patients with mantle cell lymphoma who progressed after bortezomib therapy. Blood. 2014;124(21):4471. https://doi.org/10.1182/blood.V124.21.4471.4471.
Bernard S, Goldwirt L, Amorim S, Brice P, Brière J, de Kerviler E, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126(14):1695–8. https://doi.org/10.1182/blood-2015-05-647834.
Tucker DL, Naylor G, Kruger A, Hamilton MS, Follows G, Rule SA. Ibrutinib is a safe and effective therapy for systemic mantle cell lymphoma with central nervous system involvement - a multi-centre case series from the United Kingdom. Br J Haematol. 2017;178(2):327–9. https://doi.org/10.1111/bjh.14122.
Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): a covalent bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240–52. https://doi.org/10.1124/jpet.117.242909.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659–67. https://doi.org/10.1016/s0140-6736(17)33108-2.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Durable response with single-agent acalabrutinib in patients with relapsed or refractory mantle cell lymphoma. Leukemia. 2019;33(11):2762–6. https://doi.org/10.1038/s41375-019-0575-9.
Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–9. https://doi.org/10.1182/blood.2019001160.
Trotman J, Opat S, Gottlieb D, Simpson D, Marlton P, Cull G, et al. Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow-up. Blood. 2020;136(18):2027–37. https://doi.org/10.1182/blood.2020006449.
Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, et al. Safety and activity of the investigational bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in patients with mantle cell lymphoma from a phase 2 trial. Blood. 2018;132(Supplement 1):148. https://doi.org/10.1182/blood-2018-99-117956%JBlood.
Tam CS, Wang M, Simpson D, Opat S, Cull G, Munoz J, et al. Updated safety and activity of the investigational bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in patients with mantle cell lymphoma. Blood. 2018;132(Supplement 1):1592. https://doi.org/10.1182/blood-2018-99-117224%JBlood.
Rule S, Dreyling M, Goy A, Hess G, Auer R, Kahl B, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica. 2019;104(5):e211–4. https://doi.org/10.3324/haematol.2018.205229.
Cheah CY, Chihara D, Romaguera JE, Fowler NH, Seymour JF, Hagemeister FB, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol. 2015;26(6):1175–9. https://doi.org/10.1093/annonc/mdv111.
Martin P, Maddocks K, Leonard JP, Ruan J, Goy A, Wagner-Johnston N, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–63. https://doi.org/10.1182/blood-2015-10-673145.
Jain P, Kanagal-Shamanna R, Zhang S, Ahmed M, Ghorab A, Zhang L, et al. Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol. 2018;183(4):578–87. https://doi.org/10.1111/bjh.15567.
Jain P, Kanagal-Shamanna R, Zhang S, Ok CY, Navsaria L, Nastoupil L, et al. Outcomes of relapsed mantle cell lymphoma patients after discontinuing acalabrutinib. Am J Hematol. 2021. https://doi.org/10.1002/ajh.26109.
Tam CS, Opat S, D’Sa S, Jurczak W, Lee HP, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038–50. https://doi.org/10.1182/blood.2020006844.
Wang M, Jain P, Yao Y, Liu Y, Zhao S, Hill H, et al. Ibrutinib plus rituximab (IR) followed by short course R-hypercvad/MTX in Patients (age ≤ 65 years) with previously untreated mantle cell lymphoma - Phase-II window-1 clinical trial. Blood. 2020;136(Supplement 1):35–6. https://doi.org/10.1182/blood-2020-137259.
Jain P, Yao Y, Zhao S, Liu Y, Hill H, Che Y, et al. Combination of Ibrutinib with rituximab (IR) in previously untreated older patients with mantle cell lymphoma (MCL) - a phase II clinical trial. Blood. 2020;136(Supplement 1):41–2. https://doi.org/10.1182/blood-2020-137167.
•• Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. https://doi.org/10.1016/s0140-6736(21)00224-5. Phase 1/2 trial results for the first reversible BTK inhibitor, pirtobrutinib. The trial included patients with relapsed MCL, including those who progressed on treatment with an alternative BTK inhibitor.
Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–22. https://doi.org/10.1038/nrc1323.
Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140(1):36–45. https://doi.org/10.1111/j.1365-2141.2007.06841.
Song K, Herzog BH, Sheng M, Fu J, McDaniel JM, Chen H, et al. Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma. Cancer Res. 2013;73(24):7254–64. https://doi.org/10.1158/0008-5472.Can-13-0750.
Ruan J, Martin P, Christos P, Cerchietti L, Tam W, Shah B, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132(19):2016–25. https://doi.org/10.1182/blood-2018-07-859769.
Albertsson-Lindblad A, Kolstad A, Laurell A, Raty R, Gronbaek K, Sundberg J, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood. 2016;128(14):1814–20. https://doi.org/10.1182/blood-2016-03-704023.
Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622–7. https://doi.org/10.1093/annonc/mdq626.
Zinzani PL, Vose JM, Czuczman MS, Reeder CB, Haioun C, Polikoff J, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892–7. https://doi.org/10.1093/annonc/mdt366.
Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688–95. https://doi.org/10.1200/jco.2013.49.2835.
Trneny M, Lamy T, Walewski J, Belada D, Mayer J, Radford J, et al. Lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol. 2016;17(3):319–31. https://doi.org/10.1016/s1470-2045(15)00559-8.
Wang M, Fayad L, Wagner-Bartak N, Zhang L, Hagemeister F, Neelapu SS, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13(7):716–23. https://doi.org/10.1016/s1470-2045(12)70200-0.
Jerkeman M, Hutchings M, Räty R, Wader KF, Laurell A, Christensen JH, et al. Ibrutinib-lenalidomide-rituximab in patients with relapsed/refractory mantle cell lymphoma: final results from the nordic lymphoma group MCL6 (PHILEMON) phase II trial. Blood. 2020;136(Supplement 1):36. https://doi.org/10.1182/blood-2020-133298.
Goy A, Ramchandren R, Ghosh N, Munoz J, Morgan DS, Dang NH, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019;134(13):1024–36. https://doi.org/10.1182/blood.2018891598.
Jerkeman M, Kolstad A, Niemann CU, Groenbaek K, Hutchings M, Pasanen A, et al. Venetoclax, lenalidomide and rituximab for patients with relapsed or refractory mantle cell lymphoma - data from the nordic lymphoma group NLG-MCL7 (VALERIA) phase I trial: stopping treatment in molecular remission is feasible. Blood. 2020;136(Supplement 1):15. https://doi.org/10.1182/blood-2020-133273.
Bentz M, Plesch A, Bullinger L, Stilgenbauer S, Ott G, Muller-Hermelink HK, et al. t(11;14)-positive mantle cell lymphomas exhibit complex karyotypes and share similarities with B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 2000;27(3):285–94.
Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826–33. https://doi.org/10.1200/jco.2016.70.4320.
Eyre TA, Walter HS, Iyengar S, Follows G, Cross M, Fox CP, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica. 2019;104(2):e68–71. https://doi.org/10.3324/haematol.2018.198812.
Sawalha Y, Goyal S, Switchenko JM, Romancik JT, Kamdar M, Greenwell IB, et al. Outcomes of patients with relapsed mantle cell lymphoma treated with venetoclax: a multicenter retrospective analysis. Blood. 2020;136(Supplement 1):4–6. https://doi.org/10.1182/blood-2020-138878.
Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378(13):1211–23. https://doi.org/10.1056/NEJMoa1715519.
Handunnetti SM, Anderson MA, Burbury K, Hicks RJ, Birbirsa B, Bressel M, et al. Three year update of the phase II ABT-199 (venetoclax) and ibrutinib in mantle cell lymphoma (AIM) study. Blood. 2019;134(Supplement_1):756. https://doi.org/10.1182/blood-2019-126619.
Le Gouill S, Morschhauser F, Chiron D, Bouabdallah K, Cartron G, Casasnovas O, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood. 2021;137(7):877–87. https://doi.org/10.1182/blood.2020008727.
Greenwell IB, Switchenko JM, Maddocks KJ, Kahl BS, Craig AFM, Alizadeh AA, et al. Bendamustine, obinutuzumab and venetoclax as induction therapy for untreated mantle cell lymphoma. Blood. 2020;136(Supplement 1):33–4. https://doi.org/10.1182/blood-2020-141454.
Davids MS, von Keudell G, Portell CA, Cohen JB, Fisher DC, Foss F, et al. Revised dose ramp-up to mitigate the risk of tumor lysis syndrome when initiating venetoclax in patients with mantle cell lymphoma. J Clin Oncol. 2018:Jco1800359. https://doi.org/10.1200/jco.18.00359.
Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–9. https://doi.org/10.1200/jco.2005.11.030.
Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171(1):88–95. https://doi.org/10.4049/jimmunol.171.1.88.
Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(4):667–75. https://doi.org/10.1200/jco.2005.03.108.
O’Connor OA, Moskowitz C, Portlock C, Hamlin P, Straus D, Dumitrescu O, et al. Patients with chemotherapy-refractory mantle cell lymphoma experience high response rates and identical progression-free survivals compared with patients with relapsed disease following treatment with single agent bortezomib: results of a multicentre Phase 2 clinical trial. Br J Haematol. 2009;145(1):34–9. https://doi.org/10.1111/j.1365-2141.2008.07466.
Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867–74. https://doi.org/10.1200/jco.2006.07.9665.
Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20(3):520–5. https://doi.org/10.1093/annonc/mdn656.
Furtado M, Johnson R, Kruger A, Turner D, Rule S. Addition of bortezomib to standard dose chop chemotherapy improves response and survival in relapsed mantle cell lymphoma. Br J Haematol. 2015;168(1):55–62. https://doi.org/10.1111/bjh.13101.
Friedberg JW, Vose JM, Kelly JL, Young F, Bernstein SH, Peterson D, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117(10):2807–12. https://doi.org/10.1182/blood-2010-11-314708.
Robak T, Jin J, Pylypenko H, Verhoef G, Siritanaratkul N, Drach J, et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(11):1449–58. https://doi.org/10.1016/s1470-2045(18)30685-5.
Kaplan LD, Maurer MJ, Stock W, Bartlett NL, Fulton N, Pettinger A, et al. Bortezomib consolidation or maintenance following immunochemotherapy and autologous stem cell transplantation for mantle cell lymphoma: CALGB/Alliance 50403. Am J Hematol. 2020;95(6):583–93. https://doi.org/10.1002/ajh.25783.
Doorduijn JK, Zijlstra JM, Lugtenburg PJ, Kersten MJ, Böhmer LH, Minnema MC, et al. Bortezomib maintenance after R-CHOP, cytarabine and autologous stem cell transplantation in newly diagnosed patients with mantle cell lymphoma, results of a randomised phase II HOVON trial. Br J Haematol. 2020;190(3):385–93. https://doi.org/10.1111/bjh.16567.
•• Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–42. https://doi.org/10.1056/NEJMoa1914347. Phase 2 trial results leading to FDA approval of brexucabtagene autoleucel (KTE-X19), the first CART-cell product approved for use in MCL.
Palomba ML, Gordon LI, Siddiqi T, Abramson JS, Kamdar M, Lunning MA, et al. Safety and preliminary efficacy in patients with relapsed/refractory mantle cell lymphoma receiving lisocabtagene maraleucel in transcend NHL 001. Blood. 2020;136(Supplement 1):10–1. https://doi.org/10.1182/blood-2020-136158.
Jain T, Sauter CS, Shah GL, Maloy MA, Chan J, Scordo M, et al. Safety and feasibility of chimeric antigen receptor T cell therapy after allogeneic hematopoietic cell transplantation in relapsed/ refractory B cell non-Hodgkin lymphoma. Leukemia. 2019;33(10):2540–4. https://doi.org/10.1038/s41375-019-0476-y.
Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–21. https://doi.org/10.1200/jco.2015.64.5929.
Neelapu SS, Jacobson CA, Oluwole OO, Munoz J, Deol A, Miklos DB, et al. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. 2020;135(23):2106–9. https://doi.org/10.1182/blood.2019004162.
Robinson SP, Boumendil A, Finel H, Peggs KS, Chevallier P, Sierra J, et al. Long-term outcome analysis of reduced-intensity allogeneic stem cell transplantation in patients with mantle cell lymphoma: a retrospective study from the EBMT Lymphoma Working Party. Bone Marrow Transplant. 2018;53(5):617–24. https://doi.org/10.1038/s41409-017-0067-3.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. https://doi.org/10.1056/NEJMoa1707447.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. https://doi.org/10.1056/NEJMoa1804980.
Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin Lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25(12):2305–21. https://doi.org/10.1016/j.bbmt.2019.08.015.
Wang M, Barrientos JC, Furman RR, Mei M, Barr PM, Choi MY, et al. VLS-101, a ROR1-targeting antibody-drug conjugate, demonstrates a predictable safety profile and clinical efficacy in patients with heavily pretreated mantle cell lymphoma and diffuse large B-cell lymphoma. Blood. 2020;136(Supplement 1):13–4. https://doi.org/10.1182/blood-2020-136373.
Lee HJ, Choi MY, Siddiqi T, Wierda WG, Barrientos JC, Lamanna N, et al. Cirmtuzumab, an anti-ROR1 antibody, in combination with ibrutinib: clinical activity in mantle cell lymphoma (MCL) or chronic lymphocytic leukemia (CLL) from a Phase 1/2 Study. Blood. 2020;136(Supplement 1):45–6. https://doi.org/10.1182/blood-2020-141917.
van der Horst HJ, de Jonge AV, Hiemstra IH, Gelderloos AT, Berry D, Hijmering NJ, et al. Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment. Blood Cancer J. 2021;11(2):38. https://doi.org/10.1038/s41408-021-00430-6.
Hutchings M, Carlo-Stella C, Bachy E, Offner FC, Morschhauser F, Crump M, et al. Glofitamab step-up dosing induces high response rates in patients with hard-to-treat refractory or relapsed non-Hodgkin lymphoma. Blood. 2020;136(Supplement 1):46–8. https://doi.org/10.1182/blood-2020-136044.
Bannerji R, Allan JN, Arnason JE, Brown JR, Advani R, Ansell SM, et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood. 2020;136(Supplement 1):42–3. https://doi.org/10.1182/blood-2020-136659.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jason T. Romancik declares that he has no conflict of interest.
Jonathon B. Cohen receives research funding through grants from Takeda, Novartis, AstraZeneca, LAM Pharmaceuticals, Loxo Oncology, Bristol-Myers Squibb/Celgene, Genentech, and Bioinvent; and has received consulting fees from AstraZeneca, BeiGene, Aptitude Health, Adaptive Biotechnologies, AstraZeneca, Kite/Gilead, Adicet Bio, Janssen, and Pharmacyclics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lymphoma
Rights and permissions
About this article
Cite this article
Romancik, J.T., Cohen, J.B. Sequencing of Novel Therapies for Mantle Cell Lymphoma. Curr. Treat. Options in Oncol. 22, 118 (2021). https://doi.org/10.1007/s11864-021-00907-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00907-3