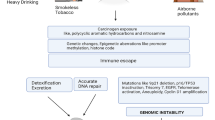
Opinion statement
Cutaneous squamous cell carcinoma (cSCC) of the head and neck is typically managed with Mohs Micrographic Surgery (MMS) for cosmetic reasons. MMS also improves oncologic outcomes for high-risk tumors. Patients with certain high-risk subsets of the disease also benefit from adjuvant radiation therapy. The PD-1 inhibitor, cemiplimab, was recently approved for treatment of locally advanced and metastatic cSCC unamenable to curative surgery or radiation therapy after the drug demonstrated encouraging, durable response rates. Cemiplimab and other systemic immunotherapies are now being evaluated in clinical trials in the neoadjuvant and adjuvant settings as well. Localized immunotherapies are also being studied, including oncolytic viruses such as talimogene laherparepvec, a modified herpes simplex virus previously approved for the treatment of advanced cutaneous melanoma. Most importantly, multidisciplinary care is crucial in optimizing outcomes for patients with high-risk cSCC of the head and neck.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–47. https://doi.org/10.1016/j.jaad.2017.08.059.
Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(1–3):8–18.
Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–83. https://doi.org/10.1056/NEJM200103293441306.
Amin MB, American Joint Committee on Cancer., American Cancer Society. AJCC cancer staging manual. Eight edition / editor-in-chief, Mahul B. Amin, MD, FCAP ; editors, Stephen B. Edge, MD, FACS and 16 others ; Donna M. Gress, RHIT, CTR - Technical editor ; Laura R. Meyer, CAPM - Managing editor. ed. Chicago: American Joint Committee on Cancer, Springer; 2017.
Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327–34. https://doi.org/10.1200/JCO.2012.48.5326.
Ruiz ES, Karia PS, Besaw R, Schmults CD. Performance of the American Joint Committee on Cancer staging manual, 8th edition vs the Brigham and Women’s Hospital tumor classification system for cutaneous squamous cell carcinoma. JAMA Dermatol. 2019;155(7):819–25. https://doi.org/10.1001/jamadermatol.2019.0032.
Breuninger H, Brantsch K, Eigentler T, Hafner HM. Comparison and evaluation of the current staging of cutaneous carcinomas. J Dtsch Dermatol Ges. 2012;10(8):579–86. https://doi.org/10.1111/j.1610-0387.2012.07896.x.
Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. https://doi.org/10.1136/bmj.f6153.
• Marrazzo G, Zitelli JA, Brodland D. Clinical outcomes in high-risk squamous cell carcinoma patients treated with Mohs micrographic surgery alone. J Am Acad Dermatol. 2019;80(3):633–8. https://doi.org/10.1016/j.jaad.2018.09.015. Large retrospective study demonstrating low rates of poor outcomes in patients with high-risk cutaneous squamous cell carcinoma treated with Mohs micrographic surgery alone.
Ad Hoc Task F, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531–50. https://doi.org/10.1016/j.jaad.2012.06.009.
Manyam BV, Joshi N, Koyhman S. A review of the role of external-beam radiation therapy in nonmelanomatous skin cancer. Appl Rad Oncol. 2017;6:6–10.
Koyfman SA, Cooper JS, Beitler JJ, Busse PM, Jones CU, McDonald MW, et al. ACR appropriateness criteria((R)) aggressive nonmelanomatous skin cancer of the head and neck. Head Neck. 2016;38(2):175–82. https://doi.org/10.1002/hed.24171.
National Comprehensive Cancer Network. Squamous cell skin cancer (Version 2.2019). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed July 30, 2019.
Veness MJ, Morgan GJ, Palme CE, Gebski V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope. 2005;115(5):870–5. https://doi.org/10.1097/01.MLG.0000158349.64337.ED.
• Harris BN, Pipkorn P, Nguyen KNB, Jackson RS, Rao S, Moore MG, et al. Association of adjuvant radiation therapy with survival in patients with advanced cutaneous squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. 2019;145(2):153–8. https://doi.org/10.1001/jamaoto.2018.3650. Large retrospective study showing association of adjuvant radiation therapy with improved survival for cutaneous squamous cell carcinoma patients with perineural invasion and patients with regional disease.
Manyam BV, Garsa AA, Chin RI, Reddy CA, Gastman B, Thorstad W, et al. A multi-institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer. 2017;123(11):2054–60. https://doi.org/10.1002/cncr.30601.
Jambusaria-Pahlajani A, Miller CJ, Quon H, Smith N, Klein RQ, Schmults CD. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35(4):574–85.
Tanvetyanon T, Padhya T, McCaffrey J, Kish JA, Deconti RC, Trotti A, et al. Postoperative concurrent chemotherapy and radiotherapy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2015;37(6):840–5. https://doi.org/10.1002/hed.23684.
Porceddu SV, Bressel M, Poulsen MG, Stoneley A, Veness MJ, Kenny LM, et al. Postoperative concurrent chemoradiotherapy versus postoperative radiotherapy in high-risk cutaneous squamous cell carcinoma of the head and neck: the randomized phase III TROG 05.01 trial. J Clin Oncol. 2018;36(13):1275–83. https://doi.org/10.1200/JCO.2017.77.0941.
O'Bryan K, Sherman W, Niedt GW, Taback B, Manolidis S, Wang A, et al. An evolving paradigm for the workup and management of high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2013;69(4):595–602 e1. https://doi.org/10.1016/j.jaad.2013.05.011.
Trodello C, Higgins S, Ahadiat O, Ragab O, In G, Hawkins M, et al. Cetuximab as a component of multimodality treatment of high-risk cutaneous squamous cell carcinoma: a retrospective analysis from a single tertiary academic medical center. Dermatol Surg. 2019;45(2):254–67. https://doi.org/10.1097/DSS.0000000000001755.
Sadek H, Azli N, Wendling JL, Cvitkovic E, Rahal M, Mamelle G, et al. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer. 1990;66(8):1692–6. https://doi.org/10.1002/1097-0142(19901015)66:8<1692::aid-cncr2820660807>3.0.co;2-y.
Jarkowski A 3rd, Hare R, Loud P, Skitzki JJ, Kane JM 3rd, May KS, et al. Systemic therapy in advanced cutaneous squamous cell carcinoma (CSCC): the Roswell Park experience and a review of the literature. Am J Clin Oncol. 2016;39(6):545–8. https://doi.org/10.1097/COC.0000000000000088.
Maubec E, Petrow P, Scheer-Senyarich I, Duvillard P, Lacroix L, Gelly J, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29(25):3419–26. https://doi.org/10.1200/JCO.2010.34.1735.
Reigneau M, Robert C, Routier E, Mamelle G, Moya-Plana A, Tomasic G, et al. Efficacy of neoadjuvant cetuximab alone or with platinum salt for the treatment of unresectable advanced nonmetastatic cutaneous squamous cell carcinomas. Br J Dermatol. 2015;173(2):527–34. https://doi.org/10.1111/bjd.13741.
William WN Jr, Feng L, Ferrarotto R, Ginsberg L, Kies M, Lippman S, et al. Gefitinib for patients with incurable cutaneous squamous cell carcinoma: a single-arm phase II clinical trial. J Am Acad Dermatol. 2017;77(6):1110–3 e2. https://doi.org/10.1016/j.jaad.2017.07.048.
Gold KA, Kies MS, William WN Jr, Johnson FM, Lee JJ, Glisson BS. Erlotinib in the treatment of recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase 2 clinical trial. Cancer. 2018;124(10):2169–73. https://doi.org/10.1002/cncr.31346.
Foote MC, McGrath M, Guminski A, Hughes BG, Meakin J, Thomson D, et al. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann Oncol. 2014;25(10):2047–52. https://doi.org/10.1093/annonc/mdu368.
Cavalieri S, Perrone F, Miceli R, Ascierto PA, Locati LD, Bergamini C, et al. Efficacy and safety of single-agent pan-human epidermal growth factor receptor (HER) inhibitor dacomitinib in locally advanced unresectable or metastatic skin squamous cell cancer. Eur J Cancer. 2018;97:7–15. https://doi.org/10.1016/j.ejca.2018.04.004.
Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78(2):249–61. https://doi.org/10.1016/j.jaad.2017.08.058.
Choi FD, Kraus CN, Elsensohn AN, Carley SK, Lehmer LM, Nguyen RT, et al. PD-1 and PD-L1 inhibitors in the treatment of non-melanoma skin cancer: a systematic review. J Am Acad Dermatol. 2019. https://doi.org/10.1016/j.jaad.2019.05.077.
Liu Y, Fitzgerald B, Perry E, Pathak A, Chao HH. Prolonged response to pembrolizumab in spindle cell squamous cell carcinoma metastatic to the central nervous system. J Investig Med High Impact Case Rep. 2019;7:2324709619850216. https://doi.org/10.1177/2324709619850216.
Oliveira LJC, Gongora ABL, Barbosa FG, Dos Anjos CH, Munhoz RR. Atypical response with bone pseudoprogression in a patient receiving nivolumab for advanced cutaneous squamous cell carcinoma. J Immunother Cancer. 2018;6(1):130. https://doi.org/10.1186/s40425-018-0444-5.
van Baar MLM, Guminski AD, Ferguson PM, Martin LK. Pembrolizumab for cutaneous squamous cell carcinoma: report of a case of inoperable squamous cell carcinoma with complete response to pembrolizumab complicated by granulomatous inflammation. JAAD Case Rep. 2019;5(6):491–4. https://doi.org/10.1016/j.jdcr.2019.04.006.
Pandey A, Liaukovich M, Joshi K, Avezbakiyev BI, O’Donnell JE. Uncommon presentation of metastatic squamous cell carcinoma of the skin and treatment challenges. Am J Case Rep. 2019;20:294–9. https://doi.org/10.12659/AJCR.913488.
Day F, Kumar M, Fenton L, Gedye C. Durable response of metastatic squamous cell carcinoma of the skin to ipilimumab immunotherapy. J Immunother. 2017;40(1):36–8. https://doi.org/10.1097/CJI.0000000000000146.
Miller DM, Faulkner-Jones BE, Stone JR, Drews RE. Complete pathologic response of metastatic cutaneous squamous cell carcinoma and allograft rejection after treatment with combination immune checkpoint blockade. JAAD Case Rep. 2017;3(5):412–5. https://doi.org/10.1016/j.jdcr.2017.06.005.
•• Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–51. https://doi.org/10.1056/NEJMoa1805131. Phase 1/II trial demonstrating encouraging, durable response rates in patients with locally advanced and metastatic cutaneous squamous cell carcinoma treated with cemiplimab monotherapy.
Annals of Oncology (2019) 30 (suppl_5): v533-v563. https://doi.org/10.1093/annonc/mdz255.
Kacew AJ, Harris EJ, Lorch JH, Haddad RI, Chau NG, Rabinowits G, et al. Chromosome 3q arm gain linked to immunotherapy response in advanced cutaneous squamous cell carcinoma. Eur J Cancer. 2019;113:1–9. https://doi.org/10.1016/j.ejca.2019.03.004.
Gross N, Ferrarotto R, Nagarajan P, Bell D, El-Naggar A, Johnson JM, et al. LBA74 Phase II study of neoadjuvant cemiplimab prior to surgery in patients with stage III/IV (M0) cutaneous squamous cell carcinoma of the head and neck (CSCC-HN). Ann Oncol. 2019;30(Supplement_5). https://doi.org/10.1093/annonc/mdz394.071.
Fromm G, de Silva S, Johannes K, Patel A, Hornblower JC, Schreiber TH. Agonist redirected checkpoint, PD1-Fc-OX40L, for cancer immunotherapy. J Immunother Cancer. 2018;6(1):149–16. https://doi.org/10.1186/s40425-018-0454-3.
Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol. 2010;28:57–78. https://doi.org/10.1146/annurev-immunol-030409-101243.
Owonikoko TK, Kumar M, Yang S, Kamphorst AO, Pillai RN, Akondy R, et al. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother. 2017;66(1):45–50. https://doi.org/10.1007/s00262-016-1918-2.
Lipson EJ, Bagnasco SM, Moore J Jr, Jang S, Patel MJ, Zachary AA, et al. Tumor regression and allograft rejection after administration of Anti-PD-1. N Engl J Med. 2016;374(9):896–8. https://doi.org/10.1056/NEJMc1509268.
Fisher J, Zeitouni N, Fan W, Samie FH. Immune checkpoint inhibitor therapy in solid organ transplant recipients: a patient-centered systematic review. J Am Acad Dermatol. 2019. https://doi.org/10.1016/j.jaad.2019.07.005.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8. https://doi.org/10.1200/JCO.2014.58.3377.
Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vaccin Immunother. 2018;14(4):839–46. https://doi.org/10.1080/21645515.2017.1412896.
Pol JG, Acuna SA, Yadollahi B, Tang N, Stephenson KB, Atherton MJ, et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. Oncoimmunology. 2019;8(1):e1512329. https://doi.org/10.1080/2162402X.2018.1512329.
LaRocca CJ, Warner SG. Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clin Transl Med. 2018;7(1):35. https://doi.org/10.1186/s40169-018-0214-5.
Wang J, Aldabagh B, Yu J, Arron ST. Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermatol. 2014;70(4):621–9. https://doi.org/10.1016/j.jaad.2014.01.857.
Chahoud J, Semaan A, Chen Y, Cao M, Rieber AG, Rady P, et al. Association between beta-genus human papillomavirus and cutaneous squamous cell carcinoma in Immunocompetent individuals-a meta-analysis. JAMA Dermatol. 2016;152(12):1354–64. https://doi.org/10.1001/jamadermatol.2015.4530.
Nichols AJ, Allen AH, Shareef S, Badiavas EV, Kirsner RS, Ioannides T. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153(6):571–4. https://doi.org/10.1001/jamadermatol.2016.5703.
Nichols AJ, Gonzalez A, Clark ES, Khan WN, Rosen AC, Guzman W, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154(8):927–30. https://doi.org/10.1001/jamadermatol.2018.1748.
Kandimalla ER, Nallagatla S, Anderson BR, Kang R. Abstract A136: AST-008, a novel TLR9 agonist SNA, induces abscopal antitumor effects in mouse tumor models. Cancer Immunol Res. 2019;7(2 Supplement):A136–A. https://doi.org/10.1158/2326-6074.cricimteatiaacr18-a136.
Doener F, Hong HS, Meyer I, Tadjalli-Mehr K, Daehling A, Heidenreich R, et al. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine. 2019;37(13):1819–26. https://doi.org/10.1016/j.vaccine.2019.02.024.
Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–22. https://doi.org/10.1056/NEJMoa1613210.
Sadeghi R, Adinehpoor Z, Maleki M, Fallahi B, Giovanella L, Treglia G. Prognostic significance of sentinel lymph node mapping in Merkel cell carcinoma: systematic review and meta-analysis of prognostic studies. Biomed Res Int. 2014;2014:489536. https://doi.org/10.1155/2014/489536.
Tejera-Vaquerizo A, Garcia-Doval I, Llombart B, Canueto J, Martorell-Calatayud A, Descalzo-Gallego MA, et al. Systematic review of the prevalence of nodal metastases and the prognostic utility of sentinel lymph node biopsy in cutaneous squamous cell carcinoma. J Dermatol. 2018;45(7):781–90. https://doi.org/10.1111/1346-8138.14342.
Lhote R, Lambert J, Lejeune J, Gottlieb J, Badaoui A, Battistella M, et al. Sentinel lymph node biopsy in cutaneous squamous cell carcinoma series of 37 cases and systematic review of the literature. Acta Derm Venereol. 2018;98(7):671–6. https://doi.org/10.2340/00015555-2942.
Koyfman SA, Joshi N, Vidimos A. Adjuvant radiotherapy in high-risk cutaneous squamous cell cancer of the head and neck in immunosuppressed patients. JAAD Case Rep. 2015;1(6):S5–7. https://doi.org/10.1016/j.jdcr.2015.09.016.
Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118(2):286–92. https://doi.org/10.1016/j.radonc.2015.12.008.
Umebayashi Y, Uyeno K, Tsujii H, Otsuka F. Proton radiotherapy of skin carcinomas. Br J Dermatol. 1994;130(1):88–91. https://doi.org/10.1111/j.1365-2133.1994.tb06889.x.
Bryant CM, Dagan R, Li Z, Morris CG, Mendenhall WM. Proton therapy for nonmelanoma skin cancers with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2017;99(2):E324–E5. https://doi.org/10.1016/j.ijrobp.2017.06.1376.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Vamsi Varra declares that he has no conflict of interest.
Timothy D. Smile declares that he has no conflict of interest.
Jessica L. Geiger has received institutional research funding from Genentech and Regeneron, and has received advisory board honoraria from Regeneron.
Shlomo A. Koyfman has received research funding from Merck and Bristol-Myers Squibb, and has received honoraria from UpToDate and Varian Medical Systems.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Head and Neck Cancer
Rights and permissions
About this article
Cite this article
Varra, V., Smile, T.D., Geiger, J.L. et al. Recent and Emerging Therapies for Cutaneous Squamous Cell Carcinomas of the Head and Neck. Curr. Treat. Options in Oncol. 21, 37 (2020). https://doi.org/10.1007/s11864-020-00739-7
Published:
DOI: https://doi.org/10.1007/s11864-020-00739-7