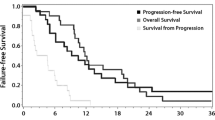
Opinion statement
T1-weighted post-contrast and T2-weighted fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) constitute the gold standard for diagnosis and response assessment in neuro-oncologic patients but are limited in their ability to accurately reflect tumor biology and metabolism, particularly over the course of a patient’s treatment. Advanced MR imaging methods are sensitized to different biophysical processes in tissue, including blood perfusion, tumor metabolism, and chemical composition of tissue, and provide more specific information on tissue physiology than standard MRI. This review provides an overview of the most common and emerging advanced imaging modalities in the field of brain tumor imaging and their applications in the care of neuro-oncologic patients.




Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Huang RY, Neagu MR, Reardon DA, Wen PY. Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy—detecting illusive disease, defining response. Front Neurol. 2015;6:33. https://doi.org/10.3389/fneur.2015.00033.
Kondziolka D, Lunsford LD, Martinez AJ. Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg. 1993;79(4):533–6. https://doi.org/10.3171/jns.1993.79.4.0533.
Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology. 2002;59(6):947–9.
White ML, Zhang Y, Kirby P, Ryken TC. Can tumor contrast enhancement be used as a criterion for differentiating tumor grades of oligodendrogliomas? AJNR Am J Neuroradiol. 2005;26(4):784–90.
• Shiroishi MS, Boxerman JL, Pope WB. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro Oncol. 2016;18(4):467–78. https://doi.org/10.1093/neuonc/nov179. A comprehensive review of applications of diffusion- and perfusion-weighted imaging.
Kalpathy-Cramer J, Gerstner ER, Emblem KE, Andronesi O, Rosen B. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res. 2014;74(17):4622–37. https://doi.org/10.1158/0008-5472.CAN-14-0383.
Barajas RF Jr, Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol. 2010;31(1):60–6. https://doi.org/10.3174/ajnr.A1750.
Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol. 2003;45(3):208–13.
Rumboldt Z, Camacho DL, Lake D, Welsh CT, Castillo M. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am J Neuroradiol. 2006;27(6):1362–9.
Lin X, Lee M, Buck O, Woo KM, Zhang Z, Hatzoglou V, et al. Diagnostic accuracy of T1-weighted dynamic contrast-enhanced-MRI and DWI-ADC for differentiation of glioblastoma and primary CNS lymphoma. AJNR Am J Neuroradiol. 2017;38(3):485–91. https://doi.org/10.3174/ajnr.A5023.
Doskaliyev A, Yamasaki F, Ohtaki M, Kajiwara Y, Takeshima Y, Watanabe Y, et al. Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3 T. Eur J Radiol. 2012;81(2):339–44. https://doi.org/10.1016/j.ejrad.2010.11.005.
Yamashita K, Yoshiura T, Hiwatashi A, Togao O, Yoshimoto K, Suzuki SO, et al. Differentiating primary CNS lymphoma from glioblastoma multiforme: assessment using arterial spin labeling, diffusion-weighted imaging, and (1)(8)F-fluorodeoxyglucose positron emission tomography. Neuroradiology. 2013;55(2):135–43. https://doi.org/10.1007/s00234-012-1089-6.
Bulakbasi N, Guvenc I, Onguru O, Erdogan E, Tayfun C, Ucoz T. The added value of the apparent diffusion coefficient calculation to magnetic resonance imaging in the differentiation and grading of malignant brain tumors. J Comput Assist Tomogr. 2004;28(6):735–46.
Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241(3):839–46. https://doi.org/10.1148/radiol.2413051276.
Wang Q, Zhang J, Xu X, Chen X, Xu B. Diagnostic performance of apparent diffusion coefficient parameters for glioma grading. J Neurooncol. 2018; https://doi.org/10.1007/s11060-018-2841-5.
Hilario A, Sepulveda JM, Perez-Nunez A, Salvador E, Millan JM, Hernandez-Lain A, et al. A prognostic model based on preoperative MRI predicts overall survival in patients with diffuse gliomas. AJNR Am J Neuroradiol. 2014;35(6):1096–102. https://doi.org/10.3174/ajnr.A3837.
Pope WB, Kim HJ, Huo J, Alger J, Brown MS, Gjertson D, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252(1):182–9. https://doi.org/10.1148/radiol.2521081534.
Pope WB, Qiao XJ, Kim HJ, Lai A, Nghiemphu P, Xue X, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108(3):491–8. https://doi.org/10.1007/s11060-012-0847-y.
Ellingson BM, Sahebjam S, Kim HJ, Pope WB, Harris RJ, Woodworth DC, et al. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR Am J Neuroradiol. 2014;35(4):673–9. https://doi.org/10.3174/ajnr.A3748.
Lee WJ, Choi SH, Park CK, Yi KS, Kim TM, Lee SH, et al. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol. 2012;19(11):1353–61. https://doi.org/10.1016/j.acra.2012.06.011.
Zeng QS, Li CF, Liu H, Zhen JH, Feng DC. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68(1):151–8. https://doi.org/10.1016/j.ijrobp.2006.12.001.
Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25(2):201–9.
Pope WB. Brain metastases: neuroimaging. Handb Clin Neurol. 2018;149:89–112. https://doi.org/10.1016/B978-0-12-811,161-1.00007-4.
White NS, McDonald C, Farid N, Kuperman J, Karow D, Schenker-Ahmed NM, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014;74(17):4638–52. https://doi.org/10.1158/0008-5472.CAN-13-3534.
Panagiotaki E, Schneider T, Siow B, Hall MG, Lythgoe MF, Alexander DC. Compartment models of the diffusion MR signal in brain white matter: a taxonomy and comparison. Neuroimage. 2012;59(3):2241–54. https://doi.org/10.1016/j.neuroimage.2011.09.081.
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–32.
Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. https://doi.org/10.1146/annurev.med.57.121304.131306.
Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology. 2006;48(3):150–9. https://doi.org/10.1007/s00234-005-0030-7.
Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–46. https://doi.org/10.1146/annurev-bioeng-071813-105,259.
Bisdas S, Kirkpatrick M, Giglio P, Welsh C, Spampinato MV, Rumboldt Z. Cerebral blood volume measurements by perfusion-weighted MR imaging in gliomas: ready for prime time in predicting short-term outcome and recurrent disease? AJNR Am J Neuroradiol. 2009;30(4):681–8. https://doi.org/10.3174/ajnr.A1465.
Spampinato MV, Schiarelli C, Cianfoni A, Giglio P, Welsh CT, Bisdas S, et al. Correlation between cerebral blood volume measurements by perfusion-weighted magnetic resonance imaging and two-year progression-free survival in gliomas. Neuroradiol J. 2013;26(4):385–95. https://doi.org/10.1177/197140091302600404.
Burth S, Kickingereder P, Eidel O, Tichy D, Bonekamp D, Weberling L, et al. Clinical parameters outweigh diffusion- and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro Oncol. 2016;18(12):1673–9. https://doi.org/10.1093/neuonc/now122.
Coban G, Mohan S, Kural F, Wang S, O’Rourke DM, Poptani H. Prognostic value of dynamic susceptibility contrast-enhanced and diffusion-weighted mr imaging in patients with glioblastomas. AJNR Am J Neuroradiol. 2015;36(7):1247–52. https://doi.org/10.3174/ajnr.A4284.
Hirai T, Murakami R, Nakamura H, Kitajima M, Fukuoka H, Sasao A, et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol. 2008;29(8):1505–10. https://doi.org/10.3174/ajnr.A1121.
Law M, Oh S, Babb JS, Wang E, Inglese M, Zagzag D, et al. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology. 2006;238(2):658–67. https://doi.org/10.1148/radiol.2382042180.
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247(2):490–8. https://doi.org/10.1148/radiol.2472070898.
Mills SJ, Patankar TA, Haroon HA, Baleriaux D, Swindell R, Jackson A. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol. 2006;27(4):853–8.
Hu LS, Eschbacher JM, Dueck AC, Heiserman JE, Liu S, Karis JP, et al. Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol. 2012;33(1):69–76. https://doi.org/10.3174/ajnr.A2743.
Jain R, Poisson L, Narang J, Gutman D, Scarpace L, Hwang SN, et al. Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology. 2013;267(1):212–20. https://doi.org/10.1148/radiol.12120846.
Cha S, Tihan T, Crawford F, Fischbein NJ, Chang S, Bollen A, et al. Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2005;26(2):266–73.
Lev MH, Ozsunar Y, Henson JW, Rasheed AA, Barest GD, GRt H, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol. 2004;25(2):214–21.
Server A, Orheim TE, Graff BA, Josefsen R, Kumar T, Nakstad PH. Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology. 2011;53(5):319–30. https://doi.org/10.1007/s00234-010-0740-3.
Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222(3):715–21. https://doi.org/10.1148/radiol.2223010558.
Liang R, Wang X, Li M, Yang Y, Luo J, Mao Q, et al. Meta-analysis of peritumoural rCBV values derived from dynamic susceptibility contrast imaging in differentiating high-grade gliomas from intracranial metastases. Int J Clin Exp Med. 2014;7(9):2724–9.
Usinskiene J, Ulyte A, Bjornerud A, Venius J, Katsaros VK, Rynkeviciene R, et al. Optimal differentiation of high- and low-grade glioma and metastasis: a meta-analysis of perfusion, diffusion. and spectroscopy metrics. Neuroradiology. 2016;58(4):339–50. https://doi.org/10.1007/s00234-016-1642-9.
Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–7. https://doi.org/10.1158/0008-5472.CAN-11-2464.
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–121. https://doi.org/10.1152/physrev.00038.2010.
Schmainda KM, Prah M, Connelly J, Rand SD, Hoffman RG, Mueller W, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16(6):880–8. https://doi.org/10.1093/neuonc/not216.
Vrabec M, Van Cauter S, Himmelreich U, Van Gool SW, Sunaert S, De Vleeschouwer S, et al. MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology. 2011;53(10):721–31. https://doi.org/10.1007/s00234-010-0802-6.
Mangla R, Singh G, Ziegelitz D, Milano MT, Korones DN, Zhong J, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256(2):575–84. https://doi.org/10.1148/radiol.10091440.
Kruser TJ, Mehta MP, Robins HI. Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother. 2013;13(4):389–403. https://doi.org/10.1586/ern.13.7.
Wan B, Wang S, Tu M, Wu B, Han P, Xu H. The diagnostic performance of perfusion MRI for differentiating glioma recurrence from pseudoprogression: a meta-analysis. Medicine (Baltimore). 2017;96(11):e6333. https://doi.org/10.1097/MD.0000000000006333.
Roberts HC, Roberts TP, Bollen AW, Ley S, Brasch RC, Dillon WP. Correlation of microvascular permeability derived from dynamic contrast-enhanced MR imaging with histologic grade and tumor labeling index: a study in human brain tumors. Acad Radiol. 2001;8(5):384–91. https://doi.org/10.1016/S1076-6332(03)80545-7.
Patankar TF, Haroon HA, Mills SJ, Baleriaux D, Buckley DL, Parker GJ, et al. Is volume transfer coefficient (K(trans)) related to histologic grade in human gliomas? AJNR Am J Neuroradiol. 2005;26(10):2455–65.
Roberts HC, Roberts TP, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol. 2000;21(5):891–9.
Choi YS, Kim DW, Lee SK, Chang JH, Kang SG, Kim EH, et al. The added prognostic value of preoperative dynamic contrast-enhanced MRI histogram analysis in patients with glioblastoma: analysis of overall and progression-free survival. AJNR Am J Neuroradiol. 2015;36(12):2235–41. https://doi.org/10.3174/ajnr.A4449.
Nguyen TB, Cron GO, Mercier JF, Foottit C, Torres CH, Chakraborty S, et al. Preoperative prognostic value of dynamic contrast-enhanced MRI-derived contrast transfer coefficient and plasma volume in patients with cerebral gliomas. AJNR Am J Neuroradiol. 2015;36(1):63–9. https://doi.org/10.3174/ajnr.A4006.
Bonekamp D, Deike K, Wiestler B, Wick W, Bendszus M, Radbruch A, et al. Association of overall survival in patients with newly diagnosed glioblastoma with contrast-enhanced perfusion MRI: comparison of intraindividually matched T1- and T2 (*)-based bolus techniques. J Magn Reson Imaging. 2015;42(1):87–96. https://doi.org/10.1002/jmri.24756.
Ulyte A, Katsaros VK, Liouta E, Stranjalis G, Boskos C, Papanikolaou N, et al. Prognostic value of preoperative dynamic contrast-enhanced MRI perfusion parameters for high-grade glioma patients. Neuroradiology. 2016;58(12):1197–208. https://doi.org/10.1007/s00234-016-1741-7.
Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol. 2014;16(2):280–91. https://doi.org/10.1093/neuonc/not148.
Rapalino O, Ratai EM. Multiparametric imaging analysis: magnetic resonance spectroscopy. Magn Reson Imaging Clin N Am. 2016;24(4):671–86. https://doi.org/10.1016/j.mric.2016.06.001.
Andronesi, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun. 2018;9:1474. https://doi.org/10.1038/s41467-018-03905-6. with permission from Nature Publishing Group
Zeng Q, Liu H, Zhang K, Li C, Zhou G. Noninvasive evaluation of cerebral glioma grade by using multivoxel 3D proton MR spectroscopy. Magn Reson Imaging. 2011;29(1):25–31. https://doi.org/10.1016/j.mri.2010.07.017.
Yang D, Korogi Y, Sugahara T, Kitajima M, Shigematsu Y, Liang L, et al. Cerebral gliomas: prospective comparison of multivoxel 2D chemical-shift imaging proton MR spectroscopy, echoplanar perfusion and diffusion-weighted MRI. Neuroradiology. 2002;44(8):656–66. https://doi.org/10.1007/s00234-002-0816-9.
Stadlbauer A, Gruber S, Nimsky C, Fahlbusch R, Hammen T, Buslei R, et al. Preoperative grading of gliomas by using metabolite quantification with high-spatial-resolution proton MR spectroscopic imaging. Radiology. 2006;238(3):958–69. https://doi.org/10.1148/radiol.2382041896.
Fountas KN, Kapsalaki EZ, Vogel RL, Fezoulidis I, Robinson JS, Gotsis ED. Noninvasive histologic grading of solid astrocytomas using proton magnetic resonance spectroscopy. Stereotact Funct Neurosurg. 2004;82(2–3):90–7. https://doi.org/10.1159/000077458.
Server A, Josefsen R, Kulle B, Maehlen J, Schellhorn T, Gadmar O, et al. Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol. 2010;51(3):316–25. https://doi.org/10.3109/02841850903482901.
Zhang H, Ma L, Wang Q, Zheng X, Wu C. Xu BN. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: a systematic review and meta-analysis. Eur J Radiol. 2014;83(12):2181–9. https://doi.org/10.1016/j.ejrad.2014.09.018.
Bluml S, Margol AS, Sposto R, Kennedy RJ, Robison NJ, Vali M, et al. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro Oncol. 2016;18(1):126–31. https://doi.org/10.1093/neuonc/nov097.
Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–84. https://doi.org/10.1007/s00401-012-0958-8.
Wilson M, Gill SK, MacPherson L, English M, Arvanitis TN, Peet AC. Noninvasive detection of glutamate predicts survival in pediatric medulloblastoma. Clin Cancer Res. 2014;20(17):4532–9. https://doi.org/10.1158/1078-0432.CCR-13-2320.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44. https://doi.org/10.1038/nature08617.
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12. https://doi.org/10.1126/science.1164382.
Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4(116):116ra4. https://doi.org/10.1126/scitranslmed.3002693.
Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–9. https://doi.org/10.1038/nm.2682.
Pope WB, Prins RM, Albert Thomas M, Nagarajan R, Yen KE, Bittinger MA, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012;107(1):197–205. https://doi.org/10.1007/s11060-011-0737-8.
• Choi C, Raisanen JM, Ganji SK, Zhang S, McNeil SS, An Z, et al. Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH-mutant glioma. J Clin Oncol. 2016;34(33):4030–9. https://doi.org/10.1200/JCO.2016.67.1222. This study demonstrated the feasibility of longitudinal 2HG MRS, its correlation with tumor grade, and a change in 2HG concentration after treatment, thus raising the possibility of using 2HG MRS as a non-invasive imaging marker of response assessment.
Jafari-Khouzani K, Loebel F, Bogner W, Rapalino O, Gonzalez GR, Gerstner E, et al. Volumetric relationship between 2-hydroxyglutarate and FLAIR hyperintensity has potential implications for radiotherapy planning of mutant IDH glioma patients. Neuro Oncol. 2016;18(11):1569–78. https://doi.org/10.1093/neuonc/now100.
Buckner J, Giannini C, Eckel-Passow J, Lachance D, Parney I, Laack N, et al. Management of diffuse low-grade gliomas in adults—use of molecular diagnostics. Nat Rev Neurol. 2017;13(6):340–51. https://doi.org/10.1038/nrneurol.2017.54.
• Andronesi OC, Loebel F, Bogner W, Marjanska M, Vander Heiden MG, Iafrate AJ, et al. Treatment response assessment in IDH-mutant glioma patients by noninvasive 3D functional spectroscopic mapping of 2-hydroxyglutarate. Clin Cancer Res. 2016;22(7):1632–41. https://doi.org/10.1158/1078-0432.CCR-15-0656. This study demonstrated the feasibility of longitudinal 2HG MRS and a change in 2HG concentration after treatment, thus raising the possibility of using 2HG MRS as a non-invasive imaging marker of response assessment.
Najac C. Ronen SM. MR molecular imaging of brain cancer metabolism using hyperpolarized 13C magnetic resonance spectroscopy. Top Magn Reson Imaging. 2016;25(5):187–96. https://doi.org/10.1097/RMR.0000000000000104.
Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158–63. https://doi.org/10.1073/pnas.1733835100.
Park I, Larson PE, Zierhut ML, Hu S, Bok R, Ozawa T, et al. Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro Oncol. 2010;12(2):133–44. https://doi.org/10.1093/neuonc/nop043.
Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci Transl Med. 2013;5(198):198ra08. https://doi.org/10.1126/scitranslmed.3006070.
Harris RJ, Cloughesy TF, Liau LM, Prins RM, Antonios JP, Li D, et al. pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro Oncol. 2015;17(11):1514–24. https://doi.org/10.1093/neuonc/nov106.
Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol. 2014;16(3):441–8. https://doi.org/10.1093/neuonc/not158.
Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ. Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res. 2017;23(14):3667–75. https://doi.org/10.1158/1078-0432.CCR-16-2265.
Goel S, England CG, Chen F, Cai W. Positron emission tomography and nanotechnology: a dynamic duo for cancer theranostics. Adv Drug Deliv Rev. 2017;113:157–76. https://doi.org/10.1016/j.addr.2016.08.001.
Chakravarty R, Hong H, Cai W. Positron emission tomography image-guided drug delivery: current status and future perspectives. Mol Pharm. 2014;11(11):3777–97. https://doi.org/10.1021/mp500173s.
Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108(5):1501–16. https://doi.org/10.1021/cr0782426.
Yoder KK. Basic PET data analysis techniques. In: Misciagna S, editor. Positron emission tomography: recent developments in instrumentation, research and clinical oncological practice. IntechOpen; 2013.
Jansen NL, Suchorska B, Wenter V, Schmid-Tannwald C, Todica A, Eigenbrod S, et al. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med. 2015;56(1):9–15. https://doi.org/10.2967/jnumed.114.144675.
Gulyas B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging. 2012;56(2):173–90.
Suchorska B, Tonn JC. Jansen NL. PET imaging for brain tumor diagnostics. Curr Opin Neurol. 2014;27(6):683–8. https://doi.org/10.1097/WCO.0000000000000143.
la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13(8):806–19. https://doi.org/10.1093/neuonc/nor054.
Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view—what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015; https://doi.org/10.1093/neuonc/nov118.
Kondo A, Ishii H, Aoki S, Suzuki M, Nagasawa H, Kubota K, et al. Phase IIa clinical study of [(18)F]fluciclovine: efficacy and safety of a new PET tracer for brain tumors. Ann Nucl Med. 2016;30(9):608–18. https://doi.org/10.1007/s12149-016-1102-y.
Parent EE, Schuster DM. Update on (18)F-fluciclovine PET for prostate cancer imaging. J Nucl Med. 2018; https://doi.org/10.2967/jnumed.117.204032.
Kunz M, Thon N, Eigenbrod S, Hartmann C, Egensperger R, Herms J, et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13(3):307–16. https://doi.org/10.1093/neuonc/noq196.
Galldiks N, Kracht LW, Dunkl V, Ullrich RT, Vollmar S, Jacobs AH, et al. Imaging of non- or very subtle contrast-enhancing malignant gliomas with [(1)(1)C]-methionine positron emission tomography. Mol Imaging. 2011;10(6):453–9.
Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–9. https://doi.org/10.1158/1078-0432.CCR-13-1440.
Galldiks N, Rapp M, Stoffels G, Fink GR, Shah NJ, Coenen HH, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33. https://doi.org/10.1007/s00259-012-2251-4.
Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49(5):694–9. https://doi.org/10.2967/jnumed.107.048082.
Kebir S, Rauschenbach L, Galldiks N, Schlaak M, Hattingen E, Landsberg J, et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 2016;18(10):1462–4. https://doi.org/10.1093/neuonc/now154.
Unterrainer M, Schweisthal F, Suchorska B, Wenter V, Schmid-Tannwald C, Fendler WP, et al. Serial 18F-FET PET imaging of primarily 18F-FET-negative glioma: does it make sense? J Nucl Med. 2016;57(8):1177–82. https://doi.org/10.2967/jnumed.115.171033.
Galldiks N, Stoffels G, Ruge MI, Rapp M, Sabel M, Reifenberger G, et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med. 2013;54(12):2046–54. https://doi.org/10.2967/jnumed.113.123836.
Romagna A, Unterrainer M, Schmid-Tannwald C, Brendel M, Tonn JC, Nachbichler SB, et al. Suspected recurrence of brain metastases after focused high dose radiotherapy: can [(18)F]FET-PET overcome diagnostic uncertainties? Radiat Oncol. 2016;11(1):139. https://doi.org/10.1186/s13014-016-0713-8.
Cicone F, Minniti G, Romano A, Papa A, Scaringi C, Tavanti F, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging. 2015;42(1):103–11. https://doi.org/10.1007/s00259-014-2886-4.
Dutour A, Kumar U, Panetta R, Ouafik L, Fina F, Sasi R, et al. Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. 1998;76(5):620–7.
Unterrainer M, Ilhan H, Todica A, Bartenstein P, Albert NL. Epidural metastases from follicular thyroid cancer mimicking meningiomas in 68Ga-DOTATATE PET. Clin Nucl Med. 2017; https://doi.org/10.1097/RLU.0000000000001793.
Rachinger W, Stoecklein VM, Terpolilli NA, Haug AR, Ertl L, Poschl J, et al. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–53. https://doi.org/10.2967/jnumed.114.149120.
Klingenstein A, Haug AR, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit. 2015;34(1):16–22. https://doi.org/10.3109/01676830.2014.959185.
Nyuyki F, Plotkin M, Graf R, Michel R, Steffen I, Denecke T, et al. Potential impact of (68)Ga-DOTATOC PET/CT on stereotactic radiotherapy planning of meningiomas. Eur J Nucl Med Mol Imaging. 2010;37(2):310–8. https://doi.org/10.1007/s00259-009-1270-2.
Graf R, Nyuyki F, Steffen IG, Michel R, Fahdt D, Wust P, et al. Contribution of 68Ga-DOTATOC PET/CT to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):68–73. https://doi.org/10.1016/j.ijrobp.2012.03.021.
Eary JF, Mankoff DA, Spence AM, Berger MS, Olshen A, Link JM, et al. 2-[C-11]Thymidine imaging of malignant brain tumors. Cancer Res. 1999;59(3):615–21.
Chen W, Cloughesy T, Kamdar N, Satyamurthy N, Bergsneider M, Liau L, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46(6):945–52.
Krause BJ, Souvatzoglou M, Treiber U. Imaging of prostate cancer with PET/CT and radioactively labeled choline derivates. Urol Oncol. 2013;31(4):427–35. https://doi.org/10.1016/j.urolonc.2010.08.008.
Bell C, Dowson N, Fay M, Thomas P, Puttick S, Gal Y, et al. Hypoxia imaging in gliomas with 18F-fluoromisonidazole PET: toward clinical translation. Seminars in nuclear medicine. 2015;45(2):136–50. https://doi.org/10.1053/j.semnuclmed.2014.10.001.
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. https://doi.org/10.1038/ncomms5006.
• Zhou M, Scott J, Chaudhury B, Hall L, Goldgof D, Yeom KW, et al. Radiomics in brain tumor: image assessment, quantitative feature descriptors, and machine-learning approaches. AJNR Am J Neuroradiol. 2018;39(2):208–16. https://doi.org/10.3174/ajnr.A5391. Review article summarizing utility and significance of radiomics in brain tumor imaging.
Parmar C, et al. Machine learning methods for quantitative radiomic biomarkers. Sci. Rep. 2015;5:13087. https://doi.org/10.1038/srep13087. with permission from Springer Nature
Kickingereder P, Gotz M, Muschelli J, Wick A, Neuberger U, Shinohara RT, et al. Large-scale radiomic profiling of recurrent glioblastoma identifies an imaging predictor for stratifying anti-angiogenic treatment response. Clin Cancer Res. 2016;22(23):5765–71. https://doi.org/10.1158/1078-0432.CCR-16-0702.
Zhou M, Chaudhury B, Hall LO, Goldgof DB, Gillies RJ, Gatenby RA. Identifying spatial imaging biomarkers of glioblastoma multiforme for survival group prediction. J Magn Reson Imaging. 2017;46(1):115–23. https://doi.org/10.1002/jmri.25497.
Macyszyn L, Akbari H, Pisapia JM, Da X, Attiah M, Pigrish V, et al. Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neuro Oncol. 2016;18(3):417–25. https://doi.org/10.1093/neuonc/nov127.
Zacharaki EI, Wang S, Chawla S, Soo Yoo D, Wolf R, Melhem ER, et al. Classification of brain tumor type and grade using MRI texture and shape in a machine learning scheme. Magn Reson Med. 2009;62(6):1609–18. https://doi.org/10.1002/mrm.22147.
Zhang Z, Yang J, Ho A, Jiang W, Logan J, Wang X, et al. A predictive model for distinguishing radiation necrosis from tumour progression after gamma knife radiosurgery based on radiomic features from MR images. Eur Radiol. 2017; https://doi.org/10.1007/s00330-017-5154-8.
Gutman DA, Cooper LA, Hwang SN, Holder CA, Gao J, Aurora TD, et al. MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology. 2013;267(2):560–9. https://doi.org/10.1148/radiol.13120118.
Gutman DA, Dunn WD Jr, Grossmann P, Cooper LA, Holder CA, Ligon KL, et al. Somatic mutations associated with MRI-derived volumetric features in glioblastoma. Neuroradiology. 2015;57(12):1227–37. https://doi.org/10.1007/s00234-015-1576-7.
Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol. 2016;61(13):R150–66. https://doi.org/10.1088/0031-9155/61/13/R150.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Ly, K.I., Gerstner, E.R. The Role of Advanced Brain Tumor Imaging in the Care of Patients with Central Nervous System Malignancies. Curr. Treat. Options in Oncol. 19, 40 (2018). https://doi.org/10.1007/s11864-018-0558-5
Published:
DOI: https://doi.org/10.1007/s11864-018-0558-5