Opinion statement
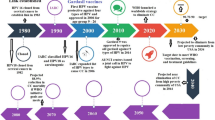
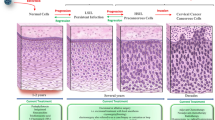
Since the publication of the American Cancer Society (ACS)/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology (ASCP) clinical guidelines in 2012, the majority of practice organizations have reached a consensus on screening recommendations for a low-risk population. These guidelines were based on a thorough review of the evidence with reproducible methods to obtain high-quality, generalizable guidelines. Despite the strength of the evidence based recommendations comprising these guidelines, limitations in physician understanding and compliance remain with respect to reaching an unscreened population and defining and caring for women who are at “high risk.” “High-risk” patients are poorly characterized but should include women with a history of a prior abnormal screening, as data has shown a subsequent increased risk of cervical intraepithelial neoplasia grade 2 (CIN2) or greater, even after treatment. These women warrant more intense screening than the general population—though there are no evidence-based guidelines for optimized screening protocols in this population. Emerging data in cervical cancer screening this year includes the FDA approval of primary high-risk human papillomavirus (HPV) testing. While the data is promising, its role in clinical practice, impact on rates of colposcopy in a non-study population, and long-term outcomes are not fully understood, and ongoing research is needed. Challenges remain in this shifting environment on the optimal interval and modality for cervical cancer screening to provide the greatest benefit in detection of precancerous lesions while minimizing the harm of overtreatment. While rapid advancements in research provide improved knowledge on how to treat and prevent this disease, it is often difficult for providers across multiple specialties to remain abreast of these changes and to educate their patients about the most current recommendations. Ultimately, provider and patient education is critical both for improving primary prevention with HPV vaccination, as well as for the uptake of evidence-based screening and management guidelines aimed at detecting and treating precancerous changes of the cervix.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Devesa S et al. Cancer incidence and mortality trends among whites in the United States, 1947–84. J Natl Cancer Inst. 1987;79:701–70.
Landis S et al. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29.
Siegel R et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Chronological history of ACS recommendations for the early detection of cancer in people without cancer symptoms. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations.
ASCUS-LSIL Triage Study (ALTS) Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol. 2003;188:1393–400.
Teoh D et al. Adherence to the 2012 national cervical cancer screening guidelines: a pilot study. Am J Obstet Gynecol. 2015;212:1–9.
Bosch F et al. The causal relationship between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65.
Walboomer J et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–29.
Saslow D et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Lower Genital Tract Dis. 2012;16:1–29. This article has spawned new practice guidelines for screening which follow stringent and reproducible criteria and have been adapted universally by the majority of preventive organizations.
Gravitt P. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–9.
McCredie M et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–34.
American College of Obstetricians and Gynecologist. Screening for cervical cancer. Clinical management guidelines for obstetricians-gynecologist. Pract Bull. 2012;131:1–18.
Patridge E, et al. Cervical cancer screening. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. 2012. http://www.trikobe.org/nccn/guideline/gynecological/english/cervical_screening.pdf.
Moyer V et al. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–92.
American Medical Association. https://www.ama-assn.org/ssl3/ecomm/PolicyFinderForm.pl?site=www.ama-assn.org&uri=/resources/html/PolicyFinder/policyfiles/HnE/H-55.971.HTM. 2015.
Broutet N, et al. Comprehensive cervical cancer control: a guide to essential practice. World Health Organization. 2014; 1-378. www.who.int
Sawaya G et al. Cervical cancer screening in average-risk women: best practice advice form the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;162:851–60.
Committee of Health Care for Underserved Women. Cervical cancer screening in low-resource settings. ACOG Committee Opinion. 2015; 624: 1-3.
Huh W et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–82. This article addresses the FDA approval of high-risk HPV for primary screening, guidelines for use, and its potential role in clinical practice.
Kitchener H et al. ARTISTIC: a randomised trial of HPV testing in primary cervical screening. Health Technol Assess. 2009;13:51.
Kulasingam S, et al. Screening for cervical cancer: a decision analysis for the U.S. Preventive Services Task Force. Rockville, MD: Agency for healthcare research and quality. 2011; AHRQ Publication No 11-05157-EF-1.
Stout N et al. Trade-offs in cervical cancer prevention: balancing benefits and risks. Arch Intern Med. 2008;168:1881–9.
Miller M et al. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol. 2003;101:29–37.
Dillner J et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:1754.
Katki H et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663–72.
Rijkaart D et al. Human papillomavirus testing for the detection of high grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomized controlled trial. Lancet Oncol. 2012;13:78–88.
Ronco G et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. This data was part of the initial results suggesting improved sensitivity with HPV-based screening suggesting this as a potential primary screening modality.
Naucler P et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. NEJM. 2007;357:1589–97.
Goldie S et al. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004;103:619–31.
Kitchener H et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer. 2011;47:864–71.
Costa S et al. Human papillomavirus (HPV) test and PAP smear as predictors of outcome in conservatively treated adenocarcinoma in situ (AIS) of the uterine cervix. Gynecol Oncol. 2007;106(1):170–6.
Datta S et al. Human papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003-2005. Ann Intern Med. 2008;148:493–500.
Melnikow J et al. Cervical intraepithelial neoplasia outcomes after treatment: long-term follow-up from the British Columbia Cohort Study. J Natl Cancer Inst. 2009;101:721–8.
Sawaya G et al. Advancing age and cervical cancer screening and prognosis. J Am Geriatr Soc. 2001;49:1499–504.
Stokes-Lampard H et al. Vaginal vault smears after hysterectomy for reasons other than malignancy: a systematic review of the literature. BJOG. 2006;113:1354–65.
Pearce K et al. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med. 1996;335:1559–62.
Ferris D et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics. 2014;134:657–65.
Chatterjee A. The next generation of HPV vaccines: nonavalent vaccine V503 on the horizon. Expert Rev Vaccines. 2014;13:1279–90.
Drolet M et al. Population level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–80.
Curtis C et al. National human papillomavirus vaccination coverage among adolescents aged 13–17 years—national immunization survey—teen, United States, 2011. MMWR. 2014;63:61–70.
Nguyen M, et al. Cervical cancer screening in immunocompromised women. Obstet Gynecol Clin N Am. 2013; 339-57.
Asch W et al. Oncologic issues and kidney transplantation: a review of frequency, mortality, and screening. Adv Chron Kidney Dis. 2014;21:106–14.
Allegretti J et al. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflamm Bowel Dis. 2015;21:1089–97.
Kim S et al. Risk of high-grade dysplasia and cervical cancer in women with systemic inflammatory diseases: a population-based cohort study. Ann Rheum Dis. 2015;74:1360–7.
Kjaer S et al. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102:1478–88.
Katki H, et al. Five-year risk of recurrence after treatment of CIN 2, CIN 3, or AIS: performance of HPV and Pap cotesting in posttreatment management. ASCCP 2013; S78-84.
Denny L et al. Human papillomavirus-based cervical cancer prevention: long term results of a randomized screening trial. J Natl Cancer Inst. 2010;102:1557–67.
Gage L et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106:1–4.
Mayrand M et al. Human papillomavirus DNA versus Papanicolaou screening test for cervical cancer. N Engl J Med. 2007;357:1579–88.
Wright T, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. 2015; 136: 189-97. Publication of the ATHENA trial led to FDA approval of high risk HPV for primary screening and led to the revision of recommendations on the use of primary HPV by multiple organizations.
Arbyn M et al. European guidelines for quality assurance in cervical cancer screening. Second edition—summary document. Ann Oncol. 2010;21:448–58.
Feldman S. Human papillomavirus testing for primary cervical cancer screening. Is it time to abandon Papanicolaou testing? JAMA. 2014;174:1539–40.
Compliance with Ethical Standards
ᅟ
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Davis, M., Feldman, S. Making Sense of Cervical Cancer Screening Guidelines and Recommendations. Curr. Treat. Options in Oncol. 16, 55 (2015). https://doi.org/10.1007/s11864-015-0373-1
Published:
DOI: https://doi.org/10.1007/s11864-015-0373-1