Abstract
Introduction
A surge in critically ill patients with respiratory failure due to Covid-19 has overwhelmed ICU capacity in many healthcare systems across the world. Given a guarded prognosis and significant resource limitations, less invasive, inventive approaches such as prone positioning (PP) of non-intubated patients with hypoxemic respiratory failure were considered.
Aims and objectives
This is a prospective observational study and the aim is to evaluate the impact of awake PP at the ward level on the oxygenation levels of patients with COVID-19. We also are investigating as secondary outcomes, the risk factors for treatment failure among awake non-intubated patients who tolerated PP compared to those who did not. The primary outcome of this trial is the change in SpO2:FiO2 (SF) ratio from admission to discharge in the participants who tolerated PP compared to those that did not. Secondary outcomes included amongst others are ICU admission rate, in-hospital mortality, and length of stay.
Methods
A total of 63 patients admitted to Beaumont Hospital (BH), Dublin between January and February of 2021 with Covid-19 requiring supplemental oxygen were recruited.
Results
A total of 47 (74%) participants were reported as tolerating and 16 (26%) as non-tolerating PP. The mean rank in the primary endpoint in the tolerating group was 38 vs. 16 in the non-tolerating. This was statistically significant (P < 0.001).
Conclusion
PP was associated with improvements in oxygenation parameters without any reported serious adverse events. A well-designed, randomised control trial, testing the efficacy of PP in non-intubated Covid-19 patients is needed, before the widespread adoption of this practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A surge in critically ill patients with respiratory failure has overwhelmed intensive care unit (ICU) capacity in many healthcare systems across the world [1, 2]. The extent of morbidity and mortality from COVID-19 has led to significant resource constraints. The less invasive, as well as inventive approach of prone positioning (PP) of non-intubated patients with hypoxemic respiratory failure has started to gather a moderate amount of evidence in the literature[3, 4]. Prone positioning of intubated patients in ICU with respiratory failure has become the standard of care since first introduced in the 1970s, and has a high quality of evidence to back this up [5,6,7]. PP of awake patients was subsequently fast-tracked without the same body of evidence to support its practice during the COVID-19 pandemic [8]. However, guidelines have been published recommending this treatment given the non-invasive and well-tolerated nature of this treatment [9]. The data is currently limited regarding the magnitude of the effect of PP on oxygenation and its ability to improve patient-centred outcomes in non-intubated COVID-19 patients [8, 10]. Currently, the largest study published to date is a retrospective cohort of 42 patients [4]. We intended to add to this evidence with local data and publish the largest sample size to date. Furthermore, the potential efficacy of PP with hypoxemic respiratory failure is yet to be tested in well-designed clinical trials [4, 10]. We present a single-centred prospective observational study to look at the effect of PP.
Study design
This prospective observational single-centre study was conducted among awake non-intubated patients with COVID-19 pneumonia requiring supplemental oxygen. All of these patients were admitted to Beaumont Hospital in Dublin during January, February and March of 2021.
Inclusion criteria
Patients were eligible for the study if they had confirmed COVID-19 pneumonia with PCR testing and required supplemental oxygen at ward level care.
Exclusion criteria
Patients were excluded from the study if they were unable to provide consent to prone, considered haemodynamically unstable (SBP < 90, RR > 40), had decreased consciousness levels (GCS < 15), persistent vomiting or any reason the patient would not be able to lie prone.
This study aimed to evaluate the impact of awake PP at the ward level on the oxygenation levels of patients with COVID-19. This was measured using the SF ratio. This is a ratio of Sp02:Fi02 and has been validated in previous studies as a surrogate marker for outcome response [11, 12]. We also investigated clinical outcomes and risk factors for treatment failure among awake non-intubated patients who tolerated PP compared to those who did not.
The sample size could not be predicted or calculated during the COVID-19 pandemic and every patient that was eligible in our centre during this pandemic was included.
Methods
PP was carried out in addition to the standard of care. This included dexamethasone, titrating supplemental oxygen therapy as required, and treating any co-morbidities that presented due to or secondary to COVID-19 pneumonia. At the time of this observational period, tocilizumab or remdesivir was not being used as the standard of care in our centre. All care was escalated to the intensive care unit (ICU) as appropriate. All patients lay prone for as long as tolerated during the day and while sleeping at night. PP could also be considered lying in the lateral position if lying completely prone was not tolerated initially. Patients were recorded to tolerate PP if they lay prone or lateral for > 8 h within 24 h. This was documented in patient charts on a proforma and verified by nursing and medical staff attending to the patients.
A protocol was devised and small education sessions were held to educate staff to explain this new protocol. The PP protocol is attached as a separate document for reference, as shown in Fig. 1. Patients were monitored until treatment failure or discharge. Treatment failure is defined as a requirement for ICU-level care or death. Sp02 was recorded on admission, before PP (considered day 0), and again on day 1, day 2, day 3 and before discharge or treatment failure. Data was collected on castor eCRF [13]. All data was taken from the patient’s medical records with consent documented. This was recorded by manual entry into the e-CRF on Ccastor. This e-CRF was manually designed to collect the appropriate variables for this study. There will be no cross-over of groups allowed. Once a patient is recorded as non-tolerated their data will be recorded in the non-tolerating group.
Statistical analysis
The primary outcome of this trial is the change in the SF ratio from admission to discharge or treatment failure. Comparisons between patients who tolerated PP against those that did not tolerate PP will be made.
The secondary outcomes are:
-
1.
Risk factors of treatment failure
-
2.
Survival analysis using the time to treatment failure
-
3.
Mean difference in length of stay
Data analysis was carried out in IMB SPSS statistics version 27.0. Unless otherwise stated, all hypothesis tests were performed using two-sided tests at the 5% significance level. All statistical analysis will be in keeping with ICG-GCP guidance [14].
Tests for normal distribution were done with a Kolmogorov–Smirnov for continuous variables. The discrete or binary variables were summarised using absolute and relative frequencies. Mann–Whitney U test and Student’s T-test were carried out to compare the means of the two groups (tolerating vs. not tolerating) for change in SF ratio. Regression modelling was used to analyse the impact of predictors on treatment failure. A p-value of < 0.05% is considered to be statistically significant.
Continuous variables were summarised using descriptive statistics including means, standard deviations, minima, maxima and quartiles. Mean rank, medians and percentiles were reported for any potentially skewed continuous variables as per the appropriate statistical test.
Results
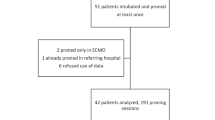
A total of 63 participants were recruited that met the inclusion criteria. Eleven patients were screened for inclusion who did not consent to participation. This sample of screened patients comprised all patients admitted to the hospital during the predefined study period under the care of the respiratory service in the respiratory wards. This was to allow for the appropriate training of healthcare staff on the prone protocol. Figure 2 demonstrates the outcome of patients in the study based on tolerability. A total of 47 (74%) participants were reported as tolerating and 16 (26%) as non-tolerating. Baseline demographics for comparison are seen in Table 1. A total of 15 participants (23%) met the criteria for treatment failure (as defined above), 9 of whom were in the non-tolerating group and 6 in the tolerating group. In the non-tolerating group, 2 people required ICU care while 4 required ICU care in the tolerating group. 8 deaths were recorded in the non-tolerating group and 3 in the tolerating group.
A Mann–Whitney U test was used to carry out comparisons of the two groups, the primary endpoint is non-parametric. The mean difference in the group that tolerated was 38 compared to 16 in the group that did not tolerate. This average treatment effect of 22 was statistically significant (P < 0.001) and has an effect size of 0.28. This is illustrated in Figs. 3 and 4. Our patient cohort population had a 0% prevalence of vaccination for COVID-19. This was due to the timing of the trial being carried out in January 2021, before the widespread availability of COVID-19 vaccines.
Binary logistic regression models were fitted to understand treatment failure and investigate potential predictors. LOS, SF on admission, SF on day 2, change in SF, age, BMI and tolerating status were included in the model. Logistic regression models reporting odds ratios (OR) with 95% confidence intervals were used to analyse the association between proning status and treatment failure, after adjustment for relevant covariates. The measure of association between an exposure and an outcome is defined as an OR. In this study, the OR is the likelihood that proning will improve SF given the individual has tolerated it compared to the odds of the outcome occurring if the individual has not tolerated it. Covariates were adjusted for those that are known to affect health including age, gender, obesity and smoking. The assumptions for the logistic regression model were tested and met appropriately.
The results can be seen in Table 2 below. Other potential predictors, including co-morbidities such as hypertension, ischaemic heart disease, heart failure, chronic kidney disease and concurrent chronic lung disease were investigated in the regression model and found to be non-significant. SF ratio on day 2, change in SF ratio and age were all statistically significant predictors (P < 0.05) with an R-squared value of 0.52. Age is a strong predictor and has an odds ratio (OR) of 1.23 (95% CI 1.03–1.38). The non-tolerating variable is the strongest predictor with an OR of 15.6 (95% CI 1.1–22.2, p < 0.05).
Using a Cox proportional hazards model, treatment failure post-admission was set as the event/hazard. A regression model was used to analyse predictors for time-to-event data. Tolerate status, BMI, age, SF ratio on admission, SF ratio on day 2 and smoking status were used in our model. Non-tolerate status, BMI, smokers, age and SF ratio on day 2 are all statistically significant predictors. The non-tolerating group is the strongest predictor with a hazard ratio of 40 (95% CI of 3.2–510.8, p-value 0.004). Length of stay was compared using Mann–Whitney U for non-parametric data. The two groups are comparable the mean in the tolerate group is 33 and the mean in the non-tolerate group is 30. This difference is not statistically significant (p = 0.6). Using a 2-sided t-test, there was no significant difference in the mean SBP on admission concerning treatment failure groups. The mean difference between the two groups was 7 mmHg and p > 0.05.
Discussion
This prospective observational study shows that the use of the prone position in awake patients has potential benefits for improving the SF ratio. This is a surrogate outcome for disease regression and improved outcomes [11, 12]. Alongside this, we have also shown a statistically significant decrease in the risk of treatment failure in those who do tolerate PP (95% CI of 3.2–510.8, p-value 0.004). Our high R-squared value of 0.52 indicates our model explains much of the variability of predictors for treatment failure. Increasing age is a statistically significant risk factor for the likelihood of treatment failure. At day 2, SF ratio on admission, BMI and age are all strong predictors for the length of stay/time to treatment failure.
This simple non-invasive technique is well-tolerated and easily implemented in the ward-based setting in hospitals. However, despite significant variability in the frequency and duration of PP and respiratory supports, PP was associated with significant improvements in oxygenation parameters without any reported serious adverse events. There was no notable increase in resources that were needed to implement this procedure. Generally, once ward staff were trained, it became easily integrated into the system. We would propose this technique be used going forward for all patients meeting the above criteria.
The APPROVE care trial by Laffey is an RCT investigating the effects of awake PP [15, 16]. This RCT was published in the Lancet in August 2021 and has demonstrated similar results in the effectiveness of awake PP on mortality benefits and treatment failure. This RCT has calculated the number needed to treat 14 to prevent one intubation. Ehrmann et al. included 24 Irish patients in total in their analysis [16]. This paper has also demonstrated strong evidence in favour of similar safety endpoints between the PP and standard care groups. This further strengthens the evidence base that PP is an effective and safe intervention.
The major limitations of our study are a lack of control arm and confounding. Confounding or selection bias plays a role as the sicker patients will be less likely to tolerate PP. This will contribute to the higher treatment failure rate in the non-tolerating group. This bias was limited by ensuring no missing data was recorded and using certain admission data such as SF ratio on admission as a covariate to control for in the appropriate regression models.
A control arm would be appropriate in a robust clinical trial design. The authors feel there is adequate equipoise to warrant this. The major limitation of this would be that it would be difficult to blind patients and/or clinicians to a control arm. Hence, this would likely be an open-labelled study. A similar design to the previous successful randomised control trials in intubated patients such as the PROSEVA trial could be implemented [6].
Conclusion
In conclusion, PP was associated with improvements in oxygenation parameters without any reported serious adverse events. Age is a risk factor for treatment failure. Patient’s BMI, SF ratio on admission and age are all predictors of length of stay. A well-designed, randomised control trial, testing the efficacy of PP in non-intubated COVID-19 patients is needed to give more robust evidence before the widespread adoption of this practice.
Data Availability
The data generated during the research and analysis are not available publicly but are available from the corresponding author on request.
References
Bellani G, Laffey JG, Pham T et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315(8)
World Health Organization (WHO) Coronavirus (COVID-19) Dashboard [Internet]. [cited 2021 Aug 11]. Available from: https://covid19.who.int/
Koeckerling D, Barker J, Mudalige NL et al (2020) Awake prone positioning in COVID-19. Thorax 75(10)
Weiss TT, Cerda F, Scott JB et al (2021) Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: a retrospective observational cohort study. Br J Anaesth 126(1)
Guerin C, Gaillard S, Lemasson S et al (2004) Effects of systematic prone positioning in hypoxemic acute respiratory failure. JAMA 292(19)
Guérin C, Reignier J, Richard JC et al (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368(23)
Scholten EL, Beitler JR, Prisk GK, Malhotra A (2017) Treatment of ARDS with prone positioning. Chest 151(1)
McNicholas B, Cosgrave D, Giacomini C et al (2020) Prone positioning in COVID-19 acute respiratory failure: just do it? Br J Anaesth 125(4)
Bamford P, Bentley A, Dean J et al (2020) ICS guidance for prone positioning of the conscious COVID patient 2020 [Internet]. [cited 2021 Aug 12]. Available from: https://emcrit.org/wp-content/uploads/2020/04/2020-04-12-Guidance-for-conscious-proning.pdf
Henderson WR, Griesdale DE, Dominelli P, Ronco JJ (2014) Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome? Can Respir J 21(4)
Khemani RG, Patel NR, Bart RD, Newth CJL (2009) Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest 135(3)
Bilan N, Dastranji A, Ghalehgolab Behbahani A (2015) Comparison of the Spo2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or acute respiratory distress syndrome. J Cardiovasc Thorac Res 7(1)
Castor EDC [Internet]. [cited 2021 Aug 11]. Available from: https://www.castoredc.com/
Ich Harmonised Tripartite Guideline (2005) Ich harmonised tripartite guideline: statistical principles for clinical trials [Internet]. [cited 2021 May 13]. Available from: https://database.ich.org/sites/default/files/E9_Guideline.pdf
Laffey J (2021) Awake prone positioning to reduce invasive ventilation in COVID-19 induced acute respiratory failure (APPROVE-CARE) [Internet]. Clinicaltrials.gov. [cited 2021 Nov 4]. Available from: https://clinicaltrials.gov/ct2/show/NCT04347941
Ehrmann S, Li J, Ibarra-Estrada M et al (2021) Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med 9(12):1387–1395
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duncan, A., Halim, D., Kholy, K.E. et al. A single-centre prospective cohort study: prone positioning in awake, non-intubated patients with covid-19 hypoxemic failure. Ir J Med Sci 192, 2351–2355 (2023). https://doi.org/10.1007/s11845-022-03259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03259-5