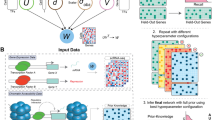
Abstract
Machine learning is increasingly recognized as a promising technology in the biological, biomedical, and behavioral sciences. There can be no argument that this technique is incredibly successful in image recognition with immediate applications in diagnostics including electrophysiology, radiology, or pathology, where we have access to massive amounts of annotated data. However, machine learning often performs poorly in prognosis, especially when dealing with sparse data. This is a field where classical physics-based simulation seems to remain irreplaceable. In this review, we identify areas in the biomedical sciences where machine learning and multiscale modeling can mutually benefit from one another: Machine learning can integrate physics-based knowledge in the form of governing equations, boundary conditions, or constraints to manage ill-posted problems and robustly handle sparse and noisy data; multiscale modeling can integrate machine learning to create surrogate models, identify system dynamics and parameters, analyze sensitivities, and quantify uncertainty to bridge the scales and understand the emergence of function. With a view towards applications in the life sciences, we discuss the state of the art of combining machine learning and multiscale modeling, identify applications and opportunities, raise open questions, and address potential challenges and limitations. We anticipate that it will stimulate discussion within the community of computational mechanics and reach out to other disciplines including mathematics, statistics, computer science, artificial intelligence, biomedicine, systems biology, and precision medicine to join forces towards creating robust and efficient models for biological systems.







Similar content being viewed by others
References
Ahmed OJ, Sudhakar SK (2019) High frequency activity during stereotyped low frequency events might help to identify the seizure onset zone. Epilepsy Curr 19(3):184–186
Ahmed OJ, John TT (2019) A straw can break a neural network’s back and lead to seizures but only when delivered at the right time. Epilepsy Currents 19(2):115–116
Alber M, Buganza Tepole A, Cannon W, De S, Dura-Bernal S, Garikipati K, Karniadakis G, Lytton WW, Perdikaris P, Petzold L, Kuhl E (2019) Integrating machine learning and multiscale modeling: perspectives, challenges, and opportunities in the biological, biomedical, and behavioral sciences. NPJ Digit Med 2:115
Ambrosi D, Ateshian GA, Arruda EM, Cowin SC, Dumais J, Goriely A, Holzapfel GA, Humphrey JD, Kemkemer R, Kuhl E, Olberding JE, Taber LA, Garikipati K (2011) Perspectives on biological growth and remodeling. J Mech Phys Solids 59:863–883
Ambrosi D, BenAmar M, Cyron CJ, DeSimone A, Goriely A, Humphrey JD, Kuhl E (2019) Growth and remodelling of living tissues: perspectives, challenges, and opportunities. J R Soc Interface 16:20190233
Anderson B, Hy TS, Kondor R (2019) ArXiv preprint arXiv:1906.04015
Athreya AP, Neavin D, Carrillo-Roa T, Skime M, Biernacka J, Frye MA, Rush AJ, Wang L, Binder EB, Iyer RK, Weinshilboum RM, Bobo WV (2019) Pharmacogenomics-driven prediction of antidepressant treatment outcomes: a machine learning approach with multi-trial replication. Clin Pharmacol Thera 106:855–865. https://doi.org/10.1002/cpt.1482
Baillargeon B, Rebelo N, Fox DD, Taylor RL, Kuhl E (2014) The living heart project: a robust and integrative simulator for human heart function. Eur J Mech A/Solids 48:38–47
Baydin AG, Pearlmutter BA, Radul AA, Siskind JM (2018) Automatic differentiation in machine learning: a survey. J Mach Learn Res 18:153
Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD (2009) Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol 5(8):593–539
Booth V, Xique IJ, Diniz Behn CG (2017) One-dimensional map for the circadian modulation of sleep in a sleep-wake regulatory network model for human sleep. SIAM J Appl Dyn Syst 16:1089–1112
Botvinick M, Ritter S, Wang JX, Kurth-Nelson Z, Blundell C, Hassabis D (2019) Reinforcement learning, fast and slow. Trends Cognit Sci 23:408–422
Brunton SL, Proctor JL, Kutz JN (2016) Discovering governing equations from data by sparse identification of nonlinear dynamical systems. Proc Natl Acad Sci 113:3932–3937
Bruynseels K, Santoni de Sio F, van den Hoven J (2018) Digital Twins in health care: ethical implications of an emerging engineering paradigm. Front Genet 9:31
Buehler MJ (2006) Atomistic and continuum modeling of mechanical properties of collagen: elasticity, fracture, and self-assembly. J Mater Res 21:19471961
Carlson KD, Nageswaran JM, Dutt N, Krichmar JL (2014) An efficient automated parameter tuning framework for spiking neural networks. Front Neurosci 8(10):00010
Cao YH, Eisenberg MC (2018) Practical unidentifiability of a simple vector-borne disease model: implications for parameter estimation and intervention assessment. Epidemics 25:89–100
Chabiniok R, Wang V, Hadjicharalambous M, Asner L, Lee J, Sermesant M, Kuhl E, Young A, Moireau P, Nash M, Chapelle D, Nordsletten DA (2016) Multiphysics and multiscale modeling, data-model fusion and integration of organ physiology in the clinic: ventricular cardiac mechanics. Interface Focus 6:20150083
Champion KP, Brunton SL, Kutz JN (2019) Discovery of nonlinear multiscale systems: sampling strategies and embeddings. SIAM J Appl Dyn Syst 18:312–333
Chandran PL, Barocas VH (2007) Deterministic material-based averaging theory model of collagen gel micromechanics. J Biomech Eng 129:137147
Chen TQ, Rubanova Y, Bettencourt J, Duvenaud DK (2018) Neural ordinary differential equations. In: Advances in neural information processing systems, pp 6571–6583
Conti S, Müller S, Ortiz M (2018) Data-driven problems in elasticity. Arch Ration Mech Anal 229:79–123
Costello Z, Martin HG (2018) A machine learning approach to predict metabolic pathway dynamics from time-series multiomics data. NPJ Syst Biol Appl 4:19
Cuperlovic-Culf M (2018) Machine learning methods for analysis of metabolic data and metabolic pathway modeling. Metabolites 8:4
De S, Wongmuk H, Kuhl E (eds) (2014) Multiscale modeling in biomechanics and mechanobiology. Springer, Berlin
Deist TM, Patti A, Wang Z, Krane D, Sorenson T, Craft D (2019) Simulation assisted machine learning. Bioinformatics 35:4072–4080. https://doi.org/10.1093/bioinformatics/btz199
Deo RC (2015) Machine learning in medicine. Circulation 132:1920–1930
DeWoskin D, Myung J, Belle MD, Piggins HD, Takumi T, Forger DB (2015) Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping. Proc Natl Acad Sci 112:E2911
Dura-Bernal S, Neymotin SA, Kerr CC, Sivagnanam S, Majumdar A, Francis JT, Lytton WW (2017) Evolutionary algorithm optimization of biological learning parameters in a biomimetic neuroprosthesis. IBM J Res Dev 61(6):114
Dura-Bernal S, Suter BA, Gleeson P, Cantarelli M, Quintana A, Rodriguez F, Lytton WW (2019) NetPyNE, a tool for data-driven multiscale modeling of brain circuits. Elife 8:e44494. https://doi.org/10.7554/eLife.44494.001
Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S (2017) Dermatologist-level classification of skin cancer with deep neural networks. Nature 542:115–118
Fritzen F, Hodapp M (2016) The finite element square reduced (FE2R) method with GPU acceleration: towards three-dimensional two-scale simulations. Int J Numer Methods Eng 107:853881
Geers MGD, Kouznetsova VG, Brekelmans WAM (2010) Multi-scale computational homogenization: trends and challenges. J Comput Appl Math 234:21752182
Gerlee P, Kim E, Anderson ARA (2015) Bridging scales in cancer progression: mapping genotype to phenotype using neural networks. Semin Cancer Biol 30:3041
Gillespie DT (2007) Stochastic simulation of chemical kinetics. Ann Rev Phys Chem 58:3555
Hagge T, Stinis P, Yeung E, Tartakovsky AM (2017) Solving differential equations with unknown constitutive relations as recurrent neural networks. arXiv:1710.02242
Han J, Jentzen A, Weinan E (2018) Solving high-dimensional partial differential equations using deep learning. Proc Natl Acad Sci 115(34):8505–8510
Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, Ng AY (2019) Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med 25:65–69
Hassabis D, Kumaran D, Summerfield C, Botvinick M (2017) Neuroscience-inspired artificial intelligence. Neuron 95(2):245258
Hicks JL, Althoff T, Sosic R, Kuhar P, Bostjancic B, King AC, Leskovec J, Delp SL (2019) Best practices for analyzing large-scale health data from wearables and smartphone apps. NPJ Digit Med 2:45
Huan X, Marzouk YM (2013) Simulation-based optimal experimental design for nonlinear systems. J Comput Phys 232:288–317
Hunt CA, Erdemir A, Lytton WW, Mac Gabhann F, Sander EA, Transtrum MK, Mulugeta L (2018) The spectrum of mechanism-oriented models and methods for explanations of biological phenomena. Processes 6(5):56
Hunter PJ, Borg TK (2003) Integration from proteins to organs: the physiome project. Processes 4:237–243
Kennedy M, O’Hagan A (2001) Bayesian calibration of computer models (with discussion). J Roy Stat Soc B 63:425–464
Kim R, Li Y, Sejnowski TJ (2019) Simple framework for constructing functional spiking recurrent neural networks. Proc Natl Acad Sci 116:22811–22820. https://doi.org/10.1101/579706
Kissas G, Yang Y, Hwuang E, Witschey WR, Detre JA, Perdikaris P (2019) Machine learning in cardiovascular flows modeling: predicting pulse wave propagation from non-invasive clinical measurements using physics-informed deep learning. ArXiv preprint arXiv:1905.04817
Kitano H (2002) Systems biology: a brief overview. Science 295:16621664
Kouznetsova V, Brekelmans WAM, Baaijens FPT (2001) Approach to micro-macro modeling of heterogeneous materials. Comput Mech 27:3748
Kouznetsova VG, Geers MGD, Brekelmans WAM (2004) Multi-scale second-order computational homogenization of multi-phase materials: a nested finite element solution strategy. Comput Methods Appl Mech Eng 193:55255550
Lange V, Picotti P, Domon B, Aebersold R (2008) Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol 4:222
Le BA, Yvonnet J, He QC (2015) Computational homogenization of nonlinear elastic materials using neural networks. Int J Numer Methods Eng 104:10611084
Leal LG, David A, Jarvelin M-R, Sebert S, Ruddock M, Karhunen V, Sternberg MJE (2019) Identification of disease-associated loci using machine learning for genotype and network data integration. Bioinformatics 35:5182–5190. https://doi.org/10.1093/bioinformatics/btz310
Leary SJ, Bhaskar A, Keane AJ (2003) A knowledge-based approach to response surface modelling in multifidelity optimization. J Glob Optim 26(3):297319
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444
Lee T, Turin SY, Gosain AK, Bilionis I, Buganza Tepole A (2018) Propagation of material behavior uncertainty in a nonlinear finite element model of reconstructive surgery. Biomech Model Mechanobiol 17(6):1857–18731
Lee T, Gosain AK, Bilionis I, Buganza Tepole A (2019) Predicting the effect of aging and defect size on the stress profiles of skin from advancement, rotation and transposition flap surgeries. J Mech Phys Solids 125:572590
Lee T, Bilionis I, Buganza Tepole A (2020) Propagation of uncertainty in the mechanical and biological response of growing tissues using multi-fidelity Gaussian process regression. Comput Methods Appl Mech Eng 359:112724
Liang G, Chandrashekhara K (2008) Neural network based constitutive model for elastomeric foams. Eng Struct 30:20022011
Lin C-L, Choi S, Haghighi B, Choi J, Hoffman EA (2018) Cluster-guided multiscale lung modeling via machine learning. In: Handbook of materials modeling, vol 120. https://doi.org/10.1007/978-3-319-50257-1_98-1
Liu Y, Zhang L, Yang Y, Zhou L, Ren L, Liu R, Pang Z, Deen MJ (2019) A novel cloud-based framework for the elderly healthcare services using Digital Twin. IEEE Access 7:49088–49101
Liu Z, Wu CT, Koishi M (2019) A deep material network for multiscale topology learning and accelerated nonlinear modeling of heterogeneous materials. Comput Methods Appl Mech Eng 345:11381168
Lu W, Su X, Klein MS, Lewis IA, Fiehn O, Rabinowitz JD (2017) Metabolite measurement: pitfalls to avoid and practices to follow. Ann Rev Biochem 86:277–304
Luebberding S, Krueger N, Kerscher M (2014) Mechanical properties of human skin in vivo: a comparative evaluation in 300 men and women. Skin Res Technol 20:127135
Lytton WW, Arle J, Bobashev G, Ji S, Klassen TL, Marmarelis VZ, Sanger TD (2017) Multiscale modeling in the clinic: diseases of the brain and nervous system. Brain Inform 4(4):219230
Lytton WW (2017) Computers, causality and cure in epilepsy. Brain 140(3):516–526
Madireddy S, Sista B, Vemaganti K (2015) A Bayesian approach to selecting hyperelastic constitutive models of soft tissue. Comput Methods Appl Mech Eng 291:102122
Madni AM, Madni CC, Lucerno SD (2019) Leveraging Digital Twin technology in model-based systems enginereering. Systems 7:1–13
Mangan NM, Brunton SL, Proctor JL, Kutz JN (2016) Inferring biological networks by sparse identification of nonlinear dynamics. IEEE Trans Mol Biol Multi-Scale Commun 2:52–63
Mangan NM, Askham T, Brunton SL, Kutz NN, Proctor JL (2019) Model selection for hybrid dynamical systems via sparse regression. Proceedings R Soc A Math Phys Eng Sci 475:20180534
Marino M, Vairo G (2012) Stress and strain localization in stretched collagenous tissues via a multiscale modelling approach. Comput Methods Biomech Biomed Eng 17:1130
McCammon JA, Gelin BR, Karplus M (1977) Dynamics of folded proteins. Nature 267:585–590
Mihai LA, Woolley TE, Goriely A (2018) Stochastic isotropic hyperelastic materials: constitutive calibration and model selection. Proc R Soc A/Math Phys Eng Sci 474:0858
Myung J, Hong S, DeWoskin D, De Schutter E, Forger DB, Takumi T (2015) GABA-mediated repulsive coupling between circadian clock neurons encodes seasonal time. Proc Natl Acad Sci 112:E2920
Nazari F, Pearson AT, Nor JE, Jackson TL (2018) A mathematical model for IL-6-mediated, stem cell driven tumor growth and targeted treatment. PLOS Comput Biol 14:e1005920. https://doi.org/10.1371/journal.pcbi.1005920
Neftci EO, Averbeck BB (2019) Reinforcement learning in artificial and biological systems. Nat Mach Intell 1:133–143
Neymotin SA, Dura-Bernal S, Moreno H, Lytton WW (2016) Computer modeling for pharmacological treatments for dystonia. Drug Discov Today Disease Models 19:5157
Neymotin SA, Suter BA, Dura-Bernal S, Shepherd GMG, Migliore M, Lytton WW (2016) Optimizing computer models of corticospinal neurons to replicate in vitro dynamics. J Neurophysiol 117:148–162
Nguyen A, Yosinski J, Clune J (2015) Deep neural networks are easily fooled: high confidence predictions for unrecognizable images. In: 2015 IEEE conference on computer vision and pattern recognition
Ognjanovski N, Broussard C, Zochowski M, Aton SJ (2018) Hippocampal network oscillations drive memory consolidation in the absence of sleep. Cereb Cortex 28(10):1–13
Park JO, Rubin SA, Amador-Noguz D, Fan J, Shlomi T, Rabinowitz JD (2016) Metabolite concentrations, fluxes and free energies imply efficient enzyme usage. Nat Chem Biol 12(7):482–489
Peirlinck M, Sahli Costabal F, Sack KL, Choy JS, Kassab GS, Guccione JM, De Beule M, Segers P, Kuhl E (2019) Using machine learning to characterize heart failure across the scales. Biomech Model Mechanobiol 18:1987–2001. https://doi.org/10.1007/s10237-019-01190-w
Peng GCY (2016) Moving toward model reproducibility and reusability. IEEE Trans Biomed Eng 63:1997–1998
Perdikaris P, Karniadakis GE (2016) Model inversion via multi-fidelity Bayesian optimization: a new paradigm for parameter estimation in haemodynamics, and beyond. J R Soc Interface 13(118):20151107
Perdikaris P, Raissi M, Damianou A, Lawrence ND, Karniadakis GE (2016) Nonlinear information fusion algorithms for robust multi-fidelity modeling. Proc R Soc A/Math Phys Eng Sci 473:0751
Poggio T, Mhaskar H, Rosasco L, Miranda B, Liao Q (2017) Why and when can deep-but not shallow-networks avoid the curse of dimensionality: a review. Int J Autom Comput 14:503–519
Proix T, Bartolomei F, Guye M, Jirsa VK (2017) Individual brain structure and modeling predict seizure propagation. Brain 140:651–654
Puentes-Mestril C, Roach J, Niethard N, Zochowski M, Aton SJ (2019) How rhythms of the sleeping brain tune memory and synaptic plasticity. Sleep zsz42:095. https://doi.org/10.1093/sleep/zsz095
Quade M, Abel M, Kutz JN, Brunton SL (2018) Sparse identification of nonlinear dynamics for rapid model recovery. Chaos 28:063116
Raina A, Linder C (2014) A homogenization approach for nonwoven materials based on fiber undulations and reorientation. J Mech Phys Solids 65:1234
Raissi M, Perdikaris P, Karniadakis GE (2017) Inferring solutions of differential equations using noisy multi-fidelity data. J Comput Phys 335:736746
Raissi M, Perdikaris P, Karniadakis GE (2017) Machine learning of linear differential equations using Gaussian processes. J Comput Phys 348:683–693
Raissi M, Perdikaris P, Karniadakis GE (2017) Physics informed deep learning (Part I): data-driven solutions of nonlinear partial differential equations. ArXiv preprint arXiv:171110561
Raissi M, Perdikaris P, Karniadakis GE (2017) Physics informed deep learning (Part II): data-driven discovery of nonlinear partial differential equations. ArXiv preprint arXiv:171110566
Raissi M, Karniadakis GE (2018) Hidden physics models: machine learning of nonlinear partial differential equations. J Comput Phys 357:125–141
Raissi M, Yazdani A, Karniadakis GE (2018) Hidden fluid mechanics: a Navier-Stokes informed deep learning framework for assimilating flow visualization data. ArXiv preprint arXiv:1808.04327
Raissi M, Perdikaris P, Karniadakis GE (2019) Physics-informed neural networks: a deep learning framework for solving forward and inverse problems involving nonlinear partial differential equations. J Comput Phys 378:686707
Rhodes SJ, Knight GM, Kirschner DE, White RG, Evans TG (2019) Dose finding for new vaccines: the role for immunostimulation/immunodynamic modelling. J Theor Biol 465:51–55
Riley P (2019) Three pitfalls to avoid in machine learning. Nature 572:27–28
Rubanova Y, Chen RTQ, Duvenaud D (2019) Latent odes for irregularly-sampled time series. ArXiv preprint arXiv:1907.03907
Rudy SH, Brunton SL, Proctor JL, Kutz JN (2017) Data-driven discovery of partial differential equations. Sci Adv 3(4):e1602614
Sahli Costabal F, Choy JS, Sack KL, Guccione JM, Kassab GS, Kuhl E (2019) Multiscale characterization of heart failure. Acta Biomater 86:66–76
Sahli Costabal F, Matsuno K, Yao J, Perdikaris P, Kuhl E (2019) Machine learning in drug development: characterizing the effect of 30 drugs on the QT interval using Gaussian process regression, sensitivity analysis, and uncertainty quantification. Comput Methods Appl Mech Eng 348:313–333
Sahli Costabal F, Perdikaris P, Kuhl E, Hurtado DE (2019) Multi-fidelity classification using Gaussian processes: accelerating the prediction of large-scale computational models. Comput Methods Appl Mech Eng 357:112602
Sahli Costabal F, Seo K, Ashley E, Kuhl E (2020) Classifying drugs by their arrhythmogenic risk using machine learning. Biophys J 118:1–12
Sahli Costabal F, Yang Y, Perdikaris P, Hurtado DE, Kuhl E (2020) Physics-informed neural networks for cardiac activation mapping. Front Phys. https://doi.org/10.3389/fphy.2020.00042
Sanchez-Lengeling B, Aspuru-Guzik A (2018) Inverse molecular design using machine learning: generative models for matter engineering. Science 361:360–365
Sander EA, Stylianopoulos T, Tranquillo RT, Barocas VH (2009) Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc Natl Acad Sci 106:1767517680
Sankaran S, Moghadam ME, Kahn AM, Tseng EE, Guccione JM, Marsden AL (2012) Patient-specific multiscale modeling of blood flow for coronary artery bypass graft surgery. Ann Biomed Eng 40(10):2228–2242
Schoeberl B, Eichler-Jonsson C, Gilles ED, Mller G (2002) Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat Biotechnol 20:370375
Shaked I, Oberhardt MA, Atias N, Sharan R, Ruppin E (2018) Metabolic network prediction of drug side effects. Cell Systems 2:209213
Shenoy VB, Miller RE, Tadmor EB, Rodney D, Phillips R, Ortiz M (1999) An adaptive finite element approach to atomic scale mechanics-the quasicontinuum method. J Mech Phys Solids 47:611642
Snowden TJ, van der Graaf PH, Tindall MJ (2017) Methods of model reduction for large-scale biological systems: a survey of current methods and trends. Bull Math Biol 79(7):14491486
Song D, Hugenberg N, Oberai AA (2019) Three-dimensional traction microscopy with a fiber-based constitutive model. Comput Methods Appl Mech Eng 357:112579
Southern J, Pitt-Francis J, Whiteley J, Stokeley D, Kobashi H, Nobes R, Kadooka Y, Gavaghan D (2008) Multi-scale computational modelling in biology and physiology. Prog Biophys Mol Biol 96:6089
Stelling J, Gilles ED (2004) Mathematical modeling of complex regulatory networks. NanoBioscience, IEEE Transactions 3:172179
Szegedy C, Zaremba W, Sutskever I, Bruna J, Erhan D, Goodfellow I, Fergus R (2013) Intriguing properties of neural networks. ArXiv preprint arXiv:1312.6199
Tank A, Covert I, Foti N, Shojaie A, Fox E (2018) Neural Granger causality for nonlinear time series. arXiv:1802.05842
Tartakovsky AM, Marrero CO, Perdikaris P, Tartakovsky GD, Barajas-Solano D (2018) Learning Parameters and constitutive relationships with physics informed deep neural networks. arXiv:1808.03398
Tartakovsky G, Tartakovsky AM, Perdikaris P (2018) Physics informed deep neural networks for learning parameters with non-Gaussian non-stationary statistics. arXiv:2018agufm.h21j1791t
Taylor CA, Figueroa CA (2009) Patient-specific modeling of cardiovascular mechanics. Annu Rev Biomed Eng 11:109134
Teichert G, Garikipati K (2019) Machine learning materials physics: surrogate optimization and multi-fidelity algorithms predict precipitate morphology in an alternative to phase field dynamics. Comput Methods Appl Mech Eng 344:666–693
Teichert GH, Natarajan AR, Van der Ven A, Garikipati K (2019) Machine learning materials physics: integrable deep neural networks enable scale bridging by learning free energy functions. Comput Methods Appl Mech Eng 353:201–216
Topol EJ (2019) Deep medicine: how artificial intelligence can make healthcare human again. Hachette Book Group, New York
Topol EJ (2019) High-performance medicine: the convergence of human and artificial intelligence. Nat Med 25:44–56
Topol EJ (2019) Deep learning detects impending organ injury. Nature 572:36–37
Tran JS, Schiavazzi DE, Kahn AM, Marsden AL (2019) Uncertainty quantification of simulated biomechanical simuli in coronary artery bypass grafts. Comput Methods Appl Mech Eng 345:402–428
Tremblay J, Prakash A, Acuna D, Brophy M, Jampani V, Anil C, Birchfield S (2018) Training deep networks with synthetic data: bridging the reality gap by domain randomization. arXiv:1804.06516
Vu MAT, Adali T, Ba D, Buzsaki G, Carlson D, Heller K, Dzirasa K (2018) A shared vision for machine learning in neuroscience. J Neurosci 38(7):16011607
Wagner GJ, Liu WK (2003) Coupling of atomistic and continuum simulations using a bridging scale decomposition. J Comput Phys 190:249274
Wang Z, Huan X, Garikipati K (2019) Variational system identification of the partial differential equations governing the physics of pattern-formation: inference under varying fidelity and noise. Comput Methods Appl Mech Eng 356:44–74
Warshel A, Levitt M (1976) Theoretical studies of enzymic reactions–dielectric, electrostatic and steric stabilization of carbonium-ion in reaction of lysozyme. J Mol Biol 103:227–249
Weickenmeier J, Kuhl E, Goriely A (2018) The multiphysics of prion-like diseases: progression and atrophy. Phys Rev Lett 121:158101
Weickenmeier J, Jucker M, Goriely A, Kuhl E (2019) A physics-based model explains the prion-like features of neurodegeneration in Alzheimers disease, Parkinsons disease, and amyotrophic lateral sclerosis. J Mech Phys Solids 124:264–281
Weinan E, Han J, Jentzen A (2017) Deep learning-based numerical methods for high-dimensional parabolic partial differential equations and backward stochastic differential equations. Commun Math Stat 5(4):349–380
Weinan E, Yu B (2018) The deep Ritz method: a deep learning-based numerical algorithm for solving variational problems. Commun Math Stat 6(1):1–12
White R, Peng G, Demir S (2009) Multiscale modeling of biomedical, biological, and behavioral systems. IEEE Eng Med 28:12–13
Wiechert W (2001) 13C metabolic flux analysis. Metab Eng 2:195–206
Wiering M, van Otterlo M (2013) Reinforcement learning and Markov decision processes. In: Reinforcement learning, pp 3–39
Wolters DA, Washburn MP, Yates JR III (2001) An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem 73(23):5683–5090
Yang L, Zhang D, Karniadakis GE (2018) Physics-informed generative adversarial networks for. arXiv:181102033 [StatML]
Yang Y, Perdikaris P (2019) Adversarial uncertainty quantification in physics-informed neural networks. J Comput Phys 394:136–152
Zangooei MH, Habibi J (2017) Hybrid multiscale modeling and prediction of cancer cell behavior. PLoS ONE 12(8):e0183810
Zhao L, Li Z, Caswell B, Ouyang J, Karniadakis GE (2018) Active learning of constitutive relation from mesoscopic dynamics for macroscopic modeling of non-Newtonian flows. J Comput Phys 363:116–127
Acknowledgements
The authors acknowledge support of the National Institutes of Health Grants U01 HL116330 (Alber), R01 AR074525 (Buganza Tepole), U01 EB022546 (Cannon), R01 CA197491 (De), U24 EB028998 (Dura-Bernal), U01 HL116323 and U01 HL142518 (Karniadakis), U01 EB017695 (Lytton), R01 EB014877 (Petzold) and U01 HL119578 (Kuhl), as well as DARPA Grant HR0011199002 and Toyota Research Institute Grant 849910, (both Garikipati). This work was inspired by the 2019 Symposium on Integrating Machine Learning with Multiscale Modeling for Biological, Biomedical, and Behavioral Systems (ML-MSM) as part of the Interagency Modeling and Analysis Group (IMAG), and is endorsed by the Multiscale Modeling (MSM) Consortium, by the U.S. Association for Computational Mechanics (USACM) Technical Trust Area Biological Systems, and by the U.S. National Committee on Biomechanics (USNCB). The authors acknowledge the active discussions within these communities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, G.C.Y., Alber, M., Buganza Tepole, A. et al. Multiscale Modeling Meets Machine Learning: What Can We Learn?. Arch Computat Methods Eng 28, 1017–1037 (2021). https://doi.org/10.1007/s11831-020-09405-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11831-020-09405-5