Abstract
Objective
To evaluate the potential efficacy of low-intensity ultrasound (US) in combination with anticancer drugs to reverse multidrug resistance (MDR) in nude mice.
Methods
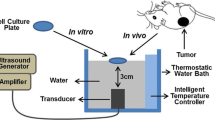
A total of 40 male and female athymic nude mice were inoculated subcutaneously with 5×106 HepG2/ADM and HepG2 cells. Ultrasound with pulsed irradiation at an average intensity of 0.5 W/cm2 was given to the tumor area 10 min after administration of adriamycin (ADM). The tumor 3 dimensional diameters were measured by calipers before and after treatment, and the tumor growth indexes (TGI) calculated. RT-PCR was used to detect the gene levels of the HepG2/ADM cells. Immunohistochemical analyses for MDR proteins were conducted on the tumor tissues.
Results
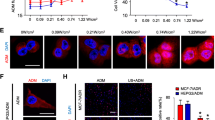
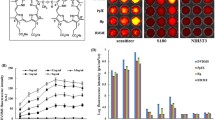
The ultrasonic treatment resulted in an average reduction in the tumor volume of 62% one month later. The relative mRNA levels of MDR1 and MRP were significantly different among the following 4 groups: untreated group as control, ADM treated; US treated; and ADM plus US treated. The mRNA levels of mdr1 and mrp were down-regulated in the US groups compared to those of the non-ultrasound groups by multiple comparisons. The relative mRNA levels of lrp expression were not significantly changed. The results of immunohistochemistry indicated that tumor tissue from animals treated with US had remarkably low mdr1 and mrp expression.
Conclusion
The results showed that low-intensity US can effectively reduce the size of adriamycin-resistant human hepotacarcinoma in a nude mouse model, and support the efficacy of US to overcome multiple mechanisms of drug resistance.
Similar content being viewed by others
References
Biedler JL.Genetic aspects of multi-drug resistance. Cancer 1992; 70: 1799–1809.
Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 1993; 62: 385–427.
Ross DD, Wooten PJ, Tong Y, et al. Synergistic reversal of multidrug-resistance phenotype in acute myeloid leukemia cells by cyclosporin A and cremophor EL. Blood 1994; 83: 1337–1347.
Malayeri R, Filipits M, Suchomel RW, et al. Multidrug resistance in leukemias and its reversal. Leuk Lymphoma 1996; 23: 451–458.
Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992; 258: 1650–164.
Scheffer GL, Wijngaard PL, Flens MJ, et al. The drug resistance-related protein LRP is the human major vault protein. Nat Med 1995; 1: 578–582.
Wu F, Wang ZB, Cao YD, et al. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer 2003; 89: 2227–2233.
Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med. Biol 1996; 22: 1131–1154.
Feigl T, Volklein B, Iro H, et al. Biophysical effects of high-energy pulsed ultrasound on human cells. Ultrasound Med. Biol 1996; 22: 1267–1275.
Liebeskind D, Bases, R, Koenigsberg, M, et al. Morphological changes in the surface characteristics of cultured cells after exposure to diagnostic ultrasound. Radiology 1981; 138: 419–423.
Nicolai H, Steinbach P, Knuechel-Clarke R, et al. Proliferation of tumor spheroids after shock-wave treatment. J Cancer Res. Clin Oncol 1994; 120: 438–441.
Zhai BJ, Shao ZY, Wu F, et al. Reversal of MDR of human hepatocarinoma HepG/adm cells by HIFU.Chinese J Cancer 2003; 22: 1284–1288.
Sundaram J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-Induced permeabilization of cell membranes. Biophys J 2003; 84: 3087–3101.
Zhai BJ, Shao ZY, Wu F, et al. Development and characterization of a multipledrug resistant human hepatoma cell Line (HepG2) in nude mouse. World J Gastroenterol 2006; 12: 6614–6619.
Krishnakumar S, Mallikarjuna K, Desai N, et al. Multidrug resistant proteins: P-glycoprotein and lung resistance protein expression in retinoblastoma. Br J Ophthalmol 2004; 88: 1521–1526.
Schondorf T, Neumann R, Benz C, et al. Cisplatin, doxorubicin and paclitaxel induce mdr1 gene transcription in ovarian cancer cell lines. Recent Results Cancer Res 2003; 161: 111–116.
Zhai BJ, Wu F, Shao ZY, et al. Overcoming multidrug resistance of human hepatorcarcinnoma by high intensity focus ultrasound in vitro. Chin J Cancer 2003; 22: 195–197 (Chinese).
Stein GH, Atkins L. Membrane-associated inhibitor of DNA synthesis in senescent human diploid fibroblasts: characterization and comparison to quiescent cell inhibitor. Proc Natl Acad Sci USA 1986; 83: 9030–9034.
Liu J, Lewis TN, Prausnitz MR. Non-invasive assessment and control of ultrasound-mediated membrane permeabilization. Pharm Res 1998; 15: 918–924.
Kremkau FW, Merritt CR, Carson PL, et al. The American Institute of Ultrasound in Medicine and the Society of Radiologists in Ultrasound—future directions in diagnostic US. Radiology 1998; 209: 305–311.
Wu J. Theoretical study on shear stress generated by microstreaming surrounding contrast agents attached to living cells. Ultrasound Med Biol 2002; 28: 125–129.
Tezel A, Mitragotri S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys J 2003; 85: 3502–3512.
Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med. Biol 1996; 22: 1131–1154.
Kawata A, Mikuni-Takagaki Y. Mechanotransduction in stretched osteocytes—temporal expression of immediate early and other genes. Biochem Biophys Res Commun 1998; 246: 404–408.
Tang H, Wang CC, Blankschtein D, et al. An investigation of the role of cavitation in low-frequency ultrasound-mediated transdermal drug transport. Pharm Res 2002; 19: 1160–1169.
Nyborg WL. Ultrasonic microstreaming and related phenomena. Br J Cancer Suppl 1982; 45: 156–160.
Webster DF, Pond JB, Dyson M, et al. The role of cavitation in the in vitro stimulation of protein synthesis in human fibroblasts by ultrasound. Ultrasound Med Biol 1978; 4: 343–351.
Pohl P, Antonenko YN, Rosenfeld E. Effects of ultrasound on the pH profiles in the unstirred layers near planar bilayer lipid membranes measured by microelectrodes. Biochim Biophys Acta 1993; 1152: 155–160.
Pohl P, Rosenfeld E, Millner R. Effects of ultrasound on the steady-state transmembrane pH gradient and the permeability of acetic acid through bilayer lipid membranes. Biochim Biophys Acta 1993; 1145: 279–283.
Matthews JC, Harder WL, Richardson WK, et al. Inactivation of firefly luciferase and rat erythrocyte ATPase by ultrasound. Membr Biochem 1993; 10: 213–220.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant from the National Natural Science Foundation of China (No. 30200060).
About this article
Cite this article
Zhai, B., Chen, L., Guo, Y. et al. Experimental study of ultrasound on MDR in a human tumor in vivo. Chin. J. Clin. Oncol. 4, 390–396 (2007). https://doi.org/10.1007/s11805-007-0390-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11805-007-0390-3