Abstract
Maritime shipping is a strategic sector with a strong international vocation and management. The need to define regulations valid for many different countries without generating disparities of treatment slowed down the formulation of environmental regulations, especially for atmospheric emissions. In particular, regulations pertaining to the reduction of sulphur compounds allowed two distinct approaches: the use of low-sulphur fuels or exhaust gas cleaning systems, the so-called Scrubbers. The actual implementation of these solutions presents specific concerns either related to the toxicity of atmospheric by-products and to the fuel cost or to the generation of polluting washwaters that may need treatment before discharge. In this paper we analyzed the potential environmental benefit deriving from the use of a distillate fuel, not compliant with current IMO Sulphur Regulations, together with a Scrubber. The pilot-scale experimental results indicated that a limited amount of water and/or scrubber volume is needed to reduce sulphur emissions below regulations on maritime shipping, especially with the addition of NaOH reaching a water-saving between 25%-33% compared to the use of pure seawater. Experiments indicated that scrubber washwater PAHs emissions are within the available water quality standards indicated by EU and USA guidelines. A bottom-up analysis on heavy metals concentration shed light on the prominent role of metal-parts corrosion on the washwater emissions. Taking into account for corrosion phenomena, the actual heavy metals concentration in the washwater deriving from scrubbing was normally below the water quality standards.
Similar content being viewed by others
1 Introduction
Due to the progressive introduction of severe regulations regarding the maximum allowed sulphur content in fuels for on-road vehicles and on the emissions of SO2 in industrial productions and power plants, the emission of SO2 in the atmosphere is progressively decreasing since more than 50 years. The maritime sector remained largely unregulated until the International Maritime Organization (IMO) introduced the Revised version of the MARPOL Annex VI guidelines (International Maritime Organization 2008). This was later ratified by many national Governments thus becoming the standard for ship emission worldwide. Until this date, the unbalancing between maritime shipping and land-based activities in terms of sulphur regulation was so large that ships were considered responsible for up to 70% of the SO2 concentration in the air in some regions in the North and Baltic Sea (Turner et al. 2017) and it was shown that SO2 emissions from shipping were around three times larger than those derived from road traffic and aviation combined (Eyring et al. 2005).
In order to achieve a reduction of sulphur emissions while preserving the overall economic balances of shipping companies and fuel availability, the MARPOL VI introduced two options to guarantee compliance to their emission targets: 1) to control the sulphur content in the fuel or; 2) to adopt exhaust gas cleaning systems to depurate the gas before they enter the atmosphere. Nowadays, the emission target imposed for all traveling ships is the equivalent emission factor, mg/kWh of sulphur equivalent to that produced by burning a fuel containing 0.5% in weight of sulphur. Besides, the same MARPOL identified some sulphur Emission Control Areas (SECA) within which the equivalent sulphur content is lowered to 0.1% in weight. When International agreements were not signed, some Regional governments impose a similar limit for ships traveling along their territorial waters, as for example in European Union (Derwent et al. 2005).
It is not easy to address properly the advantages and the problems arising from the use of a low Sulphur fuel, especially since the spirit of the original IMO guidelines was meant to provide a shift from heavy fuel oils to distillate fuels, while the market recently responded to the regulatory stimulus by inducing heavy fuel oils at low-sulphur content, whose emissions profile is still under scrutiny (Zetterdahl et al. 2016). For example, many distilled fuels were proved to be responsible for higher emissions of ultrafine particles compared with heavy fuel oils. These particles are considered highly dangerous for exposed people (Wichmann 2007; Schmid et al. 2009; Di Natale and Carotenuto 2015; Oeder et al. 2015; Reis et al. 2018; Liu et al. 2019).
Seawater scrubbers are considered the most efficient and cost-effective gas cleaning systems to remove sulphur compounds from marine diesel exhaust gases. These units used the natural alkalinity of seawater, sometimes improved by the addition of NaOH, to absorb sulphur dioxide from the exhaust gases. This process is used for around one century in coal-fired power plants and its design is well consolidated in the engineering companies. While these units can provide emission of sulphur dioxide well below the target limits, their main drawback is the transfer of other pollutants to the seawater. To this, the IMO introduced guidelines to control the quality of scrubber washwater discharge (International Maritime Organization 2006).
While complying with the reference IMO regulations, several shipping companies performed voluntarily monitoring campaigns which revealed levels of pollutants emission below wastewater regulations for direct discharge in natural waters for similar (FGD scrubbers, municipal waste incinerator, power plants) installations (Hufnagl et al. 2005; Lange and Markus 2015; DNV-GL and Carnival corporation, 2019). Together with the high dilution occurring around the ship’s keel (e.g. Lange and Markus 2015), these results indicated a low level of water pollution associated with scrubber washwater.
Nevertheless, these evaluations did not attempt to assess the cumulative effect of washwater parameters entering seawater or the potential environmental impact from these emissions. Recently several studies have raised concerns about a negative impact on the marine environment where authors pointed out how these guidelines look inappropriate and claimed for stricter regulations to control, in particular, for the emissions of persistent pollutants as PAH and heavy metals from seawater scrubbers (Turner et al. 2017, 2018; Ytreberg et al. 2019; Dulière et al. 2020). Teuchies et al. (Teuchies et al. 2020) showed that scrubber washwater emissions should be discouraged in areas with high levels of pollutions since the amount of emitted pollutants sum to the already polluted background. This conclusion holds true, however, for all the existing sources of pollutants active in that area.
These publications have led to an extended discussion within the IMOs Sub-Committee on Pollution Prevention & Response (PPR) while some port and regional authorities have banned the use of open-loop scrubbers (International Maritime Organization 2015).
Indeed, PAH and heavy metals derive from the use of carbon-based fuels and are normally emitted in the atmosphere, where they react or disperse, being eventually deposited over the nearby sea or land. When a scrubber is adopted, it is concentrated in a limited amount of water and transferred from the air to the sea. Whether it is better to emit these pollutants in the air or the sea, is a question that requires further investigations.
Independently from the actual fate of emitted pollutants, it is clear that the heavier the fuels the higher the tendency to have PAH and heavy metals in the gas exhausts. Therefore, to reduce ship emissions, lighter fuels should be used. Marine fuels are currently classified according to the ISO 8217:2017 and more recent ISO/PAS 23,263:2019, which indicates two main classes of fuels: marine distillates and marine residual fuels. The first is characterized, among the other properties, by low levels of ashes (< 0.01% in weight) and carbon micro residues (< 0.30% in weight) while the others have different qualities according to the average molecular weight of its constituents (ashes from 0.04% to 0.15% in weight; carbon micro residue from 2.5% to 20% in weight).
Notwithstanding their better properties, not all distillate fuels are able to comply with regulations on sulphur content and the toxicity of the particles they produce in the marine engines appeared even larger than those produced by residual fuels (Wichmann 2007; Schmid et al. 2009; Di Natale and Carotenuto 2015; Oeder et al. 2015; Reis et al. 2018; Liu et al. 2019). In this sense, the use of scrubbers, with their capacity to remove sulphur compounds and partially capture particles (e.g. (Lehtoranta et al. 2019; Winnes et al. 2020)) and part of the PAHs (Winnes et al. 2020) shifting them to the washwater and making them harmless from humans and the environment, is a valuable option to reduce the environmental footprint of ships, as suggested in recent studies (Ha et al. 2010; Tang et al. 2014; Di Natale et al. 2015, 2018, 2019a, 2020a, b; Darake et al. 2016; Flagiello et al. 2017, 2018a, b, 2019a, b, 2020a, b, c; Schultes et al. 2018; Iliuta and Iliuta 2019; Iliuta and Larachi 2019; Wang et al. 2019; Kuang et al. 2020; Flagiello 2020).
This paper proposes a possible option to reduce ships’ environmental footprint both in terms of air and water impact by using a distillate fuel in combination with a marine scrubber. This kind of fuel can be used easily in most of the existing engines and are largely available on the market. Similarly, the marine scrubber market largely consolidated in the last years, with several suppliers available worldwide.
To this end, this work reported experimental results on a pilot-scale seawater scrubber used to treat a 4-stroke marine diesel engine fueled with a 0.92% sulphur-fuel Marine Gas Oil (MGO). The experiments include both an analysis of gas pollutants removal efficiency and of the concentration of the PAH and the heavy metals in the scrubber washwater. The results are compared with the global and the SECA limits on sulphur emissions imposed by the IMO and with the limits imposed on PAH and heavy metals for land-based installation and with the indication for Natural Waters Quality Standards imposed by several countries.
2 Materials and Methods
2.1 Materials
The use of seawater as scrubbing liquid is related to the natural abundance of dissolved alkaline species in seawater compared to freshwater, which shifts the chemical equilibria towards a greater dissociation of H2SO3 (Iliuta and Larachi 2001; Rodríguez-Sevilla et al. 2004; Andreasen and Mayer 2007; Darake et al. 2016; Flagiello et al. 2018a, b, 2019a, b, c), as shown in Eqs. (1)‒(6):
This larger dissociation of H2SO3 occurring under alkaline conditions gives rise to a significant improvement of SO2 solubility in water: SO2 dissolves integrally in water as long as there are enough HCO3− and CO32− ions to complete the reactions (Rodríguez-Sevilla et al. 2004; Andreasen and Mayer 2007; Flagiello et al. 2017, 2018a, 2019b).
In the Mediterranean Sea and in the Oceans, the alkalinity of water is quite high: 2.3–2.4 mmol/L (Lee et al. 2006), allowing an appreciable chemical absorption of sulphur dioxide. On contrary, the Baltic Sea and North Pacific have lower alkalinity. To this hand, a limited amount of NaOH is added to the natural seawater to improve the absorption of sulphur dioxide while containing the risks of precipitation of hydroxides in seawater. The typical reaction mechanism of SO2 in an aqueous solution containing OH− ions is shown below:
where sulphur dioxide once absorbed from gas-phase in the aqueous-phase (SO2(aq)) is hydrolyzed to HSO3− and subsequently into SO32−.
In this work, the experiments were carried out using the exhaust gas produced by a four-cylinder diesel engine fed with a Marine Gas Oil (MGO) fuel containing 0.92% in weight of sulphur, whose chemical composition is shown in Table 1. The engine oil used at the test bench was a Volvo Penta VDS-3.
The FGD experiments were carried out using a sample of seawater taken from the sea area of Kattegat, located between the Jutlandic peninsula in the west, the Danish Straits islands of Denmark in the south, and the Sweden coasts in the east. This water is indicated as KSW (Kattegat Seawater) in the following and its composition is shown in Tables 2 and 3. The same seawater was eventually doped with 100 mg/L of NaOH until reaching pH = 10.5, to simulate typical water used for scrubbing in the Baltic Sea (Tang et al. 2014; Wang et al. 2019; Kuang et al. 2020). This last water solution is named KSWOH in this paper.
The sodium hydroxide used was purchased from VWR International Chemicals (Sweden) as AR grade (99.99% in weight) to enhance the seawater of Kattegat (see Eqs. (7)‒(8)).
2.2 Experimental Set-Up
The experiments were carried out in the Engine lab of the Department of Mechanics and Maritime Sciences, Chalmers University of Technology (Göteborg, Sweden), on a Volvo PENTA four-cylinder 80 kW engine marine diesel engine (D3-110) operated by fixing three different loads at 2000 r/min corresponding to engine loads at 10%, 25% and 50% of the rated maximum load.
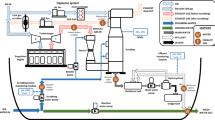
The pilot-scale spray tower (i.d.: 400 mm and total length: 500 mm) was made in stainless steel AISI 316L and was positioned horizontally, unlike the common vertical scrubbers. The system was operated at atmospheric pressure. The flowsheet of the experimental plant is shown in Figure 1.
Different engine loads can be tested by varying the engine speed and the hydraulic torque. The load variation influences the fuel consumption, temperature, and composition of the flue-gas generated.
A PLC unit allowed to manage the engine rotation per minute, the hydraulic torque, the cooling water and to measure the temperature and pressure of the engine and the support units. The operating conditions collected during operations were resumed in Table 4.
On the outlet line of the Volvo Penta diesel engine exhausts, a solenoid valve allows to split the gas flow rate into two streams: the first was sent to the engine lab chimney and the second one was sent to the scrubber in order to be treated.
The gas flow rate sent to the scrubber was controlled by using the solenoid valve on the exhaust pipe. The piping connecting the engine to the spray tower was made in Stainless Steel AISI 316L (i.d. 74 mm) and was thermally insulated to reduce the heat losses.
The flue-gas was fed in counter-current flow to the water into the scrubber. The flow rate, temperature, and pressure of the flue-gas after the scrubber were measured by a portable analyzer (Testo 480 Multi-function) equipped with a steel Pitot tube inserted into the outlet scrubber piping.
The liquid flow was fed by a frequency-controlled progressing cavity pump (Getriebebau Nord GmbH & Co.KG, D-22941 model, with a total power of 1.8 kW) and its pressure was controlled by a pressure gauge 0–10 bar (WIKA Instruments, 233.50.63 model). The liquid flow was measured by ROTA Yokogawa liquid rotameter 0–10 L/min before the scrubber. A BETE® spray nozzle (HA 1.50–9020 model, made in stainless steel) was placed at 140 mm of distance from the scrubber inlet. In order to reduce the amount of water dragged by the gas, two honeycomb grid demisters made in stainless steel AISI 316L (i.d.: 400 mm) were put at 140 mm from the scrubber outlet. Six different liquid flow rates (L) between 30 and 180 L/h available at 20 °C, were tested. The washwater was stored in a steel tank of about 5 m3 and then disposed of.
The gas analysis system was a Fuji Electric ZRE type NDIR gas analyzer (SO2, NO, CO and CO2) and Ankersmid Sampling (AOX 100 model) NOx converter. This unit allowed to measure the total NOx level with Fuji Electric ZRE, while if it was by-passed it only provided the reading of the NO emissions. O2 was measured using a paramagnetic oxygen sensor (PAROX 1200). Flue-gas sample was previously cleaned from the soot with hot filter (J.U.M. Engineering, heated sample filter 1128 model) and dehumidified with a gas quencher (Ankersmid Sampling gas cooler) at low temperature.
2.3 Experimental Procedure
The pilot scrubber was fed with a flue-gas stream with two different flow rates (G): in the first experimental set, it was constant at 70 m3/h (0.15 m/s), while in the second it was double, 140 m3/h (0.31 m/s). The characteristics and physical properties of the flue-gas deriving from the Volvo diesel engine were reported in the former Table 4. The input concentrations of the gas pollutants were determined by a gas analyzer, feeding flue-gas into the scrubber without the liquid feed.
The seawater stream (L) was sent in counter-current flow with the gas at the different flow rates, from 30 to 180 L/h. The liquid was available at room temperature in June (20 °C), either as pure seawater (Kattegat seawater, KSW) or with additions of 100 mg/L of NaOH (KSWOH).
The scrubber output concentration levels of gas pollutants were monitored and recorded up for about 10 min until reaching stable measurement conditions (i.e. steady-state), which corresponded to a deviation of more or less than 5% on the concentration measurement and of about 1 part per million when the concentration values were lower than 20 parts per million. The system behaved stably in all experimental tests, but the data reproducibility is limited by the specific functioning of the engine.
The washwater sample was taken from the bottom of the scrubber and the pH value and temperature were measured soon by a portable pH-meter (pH Tester 30 with accuracy ± 0.1 for pH). For some water samples, the ionic, organic, and heavy metals composition was also analyzed by an accredited chemical analysis laboratory, according to ISO standards.
3 Results and Discussion
3.1 Gas Pollutant Emissions
Figures 2 and 3 show the SO2 emissions at the scrubber exit as a function of the liquid–gas mass ratio (L/G) for KSW and KSWOH. The results are referred to two different flue-gas flow rates, equal to 70 and 140 m3/h and for three different engine loads: 10%, 25%, and 50%. The lines denote the maximum allowed SO2 emissions in SECAs and open sea (GLOBAL) calculated on the basis of the specific fuel consumptions and the sulphur content of the fuel.
Experimental SO2 outlet concentrations from FGD pilot unit with 70 m3/h of flue-gas stream as a function of the L/G and parametric with different engine load (10%, 25% and 50%) and different scrubbing solutions at 20 °C (KSW and KSWOH). The horizontal lines indicate the SO2 corresponding to the GLOBAL and SECA targets. L/G = 0 indicate the SO2 outlet from the scrubber turned off
Experimental SO2 outlet concentrations from FGD pilot unit with 140 m3/h of flue-gas stream as a function of the L/G and parametric with different engine load (10%, 25% and 50%) and different scrubbing solutions at 20 °C (KSW and KSWOH). The horizontal lines indicate the SO2 corresponding to the GLOBAL and SECA targets. L/G = 0 indicate the SO2 outlet from the scrubber turned off
Figures 2 and 3 show how much liquid is required to comply with the global and SECA emission targets for the two-gas flow rate and for the two kinds of seawater. In general, the L/G ratio increases with the engine load, due to the higher concentration of SO2 in the gas stream.
Besides, the experiment showed that, compared with the simple KSW, the KSWOH solution allowed a water-saving of between 7 and 50% to meet the global IMO limit and between 25 and 33% for the SECA limit. It is also worth noticing that the increase of gas flow rate worsened the SO2 removal. This is probably since most of the scrubbing takes place at the scrubber entrance so that although the gas residence time halved passing from 70 to 140 m3/h, the overall absorption was scarcely unaltered.
Table 5 shows the inlet/outlet emissions of SO2 other gas pollutants (NOx, CO and CO2) with the gas inlet/outlet temperature as a function of the engine loads and liquid–gas mass ratio (L/G) for KSW and KSWOH solutions.
The measured concentrations of NOx, CO and CO2 in line with earlier experiments performed with this engine setup (Anderson et al. 2015). As expected, the results showed that seawater scrubbing has negligible effects on the removal of NOx, CO and CO2 also when NaOH was added to the liquid. This result mirrored the low solubility of these gases in the seawater acidified by the absorption of SO2 and why SWS is not commonly used to reduce emissions of these gases. Table 5 also indicated that the gas temperatures decreased due to the contact with the cold scrubbing liquid, fed at 20 °C and that no effect of the limited heat of absorption associated with SO2 solubilization, which was the only gas pollutant absorbed was to be observed. Despite the inlet temperatures were always higher when the load increased, the outlet temperatures were very similar, with a maximum deviation between 10 and 20 °C when the load is increased at 140 m3/h.
In a former paper (Anderson et al. 2015) the particle emissions of the same engine unit were analyzed for four different fuels. Independently of fuel, bimodal size distributions by number were shown, with peaks at 10 nm and 45–50 nm for distillates and 10 and 100–110 nm for Heavy Fuel Oil (HFO). The emissions of nanoparticles with a size below 50 nm were the dominating fraction of the total particle number concentration and can be related to both sulfur content and other properties of the fuel.
The experiments showed lower emissions of particles larger than 50 nm and for volatile aerosols for distillate fuels compared with residual fuels. Recently Lethoranta (Lehtoranta et al. 2019) and Winnes and Fridell (Winnes et al. 2020) showed that a conventional scrubber applied on a residual fuel reduces up to 50% of particles mass and may remove from 10 to 80% of particles number, but better reduction of mass emissions (mg/kWh) achieved for distilled fuels (around 50%). The scrubber also removed a portion (35%–60%) of the PAHs normally emitted in the atmosphere (Winnes et al. 2020). The joint action of distilled fuels and scrubber is expected to reduce particle emissions around 75%.
Further reductions of particulate matter can be achieved using electrified scrubbers that were proved to reduce diesel engines particles emissions fueled with distilled and residual fuels by more than 85%–90% in number and mass (Carotenuto et al. 2010; Ha et al. 2010; Di Natale et al. 2015, 2016, 2020a). The same electrification is also able to increase the absorption efficiency of sulphur dioxide (Di Natale et al. 2019a, b, 2020b).
3.2 Washwaters Properties
An analysis of the pH and temperatures of the washwater was performed for each FGD test described in the previous section. Although turbidity and total suspended solids (TSS) were not measured, the experimental observations showed a limited increase of turbidity with the presence of fine suspended black particles on the water surface and a smell of sulphur dioxide, which increased as the engine load increased.
Figures 4 and 5 show the pH values of the washwater at the scrubber outlet as a function of the liquid–gas mass ratio for KSW and KSWOH in the different operating conditions, i.e. flue-gas flow rates and engine loads.
The pH values of the washwater in Figures 4 and 5 were consistent with the SO2 emission trends. As the engine load increased, the ranges of pH values in the washwaters lowered because more SO2 was captured. It is worth noting that the seawater samples gave rise to an increase of pH when the complete SO2 removal was achieved, in particular for all the conditions investigated for a flow rate of 70 m3/h of flue-gas. Generally, the use of NaOH allowed an increase of pH by at most one pH unit because absorption completed when the water alkalinity and hydroxide content were consumed. The pH values of effluents after the scrubber were in the range 3–5, which is usually considered high enough to guarantee a pH higher than 6.5 at 4 m from the discharge point, as suggested by IMO guidelines 2009, Resolution MEPC 184(59), (Gregory and Confuorto 2012; Endres et al. 2018; IMO (International Maritime Organization) MEPC 74/INF.10, 2019). Generally, the amount of water needed to restore the pH within 4 m is about 1.9 times higher than that used for gas cleaning process (United States Environmental Protection Agency, 2011) and dilution around the keel is fast enough to easily achieve this goal when pH is above 3.5 (e.g. Lange and Markus 2015).
Figures 6 and 7 show the temperature of effluents at the scrubber outlet as a function of the liquid–gas mass ratio for KSW and KSWOH in the same different operating conditions reported in Figures 4 and 5.
Figures 6 and 7 showed that the liquid temperature remained almost unvaried with the engine load. This was probably due to the heat losses of the scrubber unit accompanied by the low energy of absorption of SO2 in water. When the flue-gas flow rate was 140 m3/h the exit liquid temperature is almost 5 °C higher than in the case of lower gas flow rate.
The temperature of scrubber washwater was generally in the range 30–50 °C.
Effluent discharges with temperatures above 40 °C can cause eutrophication effects, as suggested by IMO guidelines 2009 (Resolution MEPC 184(59)). However, the same mixing with fresh seawater, made to restore the pH (United States Environmental Protection Agency, 2011), is sufficient to cool down the scrubber washwater below critical levels.
3.3 FGD Effect on Heavy Metals and Organics Emissions in Washwaters
To evaluate the effect of the FGD process on heavy metals and organics emissions in the scrubber washwaters, further chemical analyses were performed on three washwater samples collected under the following conditions:
-
SAMPLE 1:
engine load = 10%; G = 70 m3/h; L = 60 L/h;
-
SAMPLE 2:
engine load = 25%; G = 70 m3/h; L = 120 L/h;
-
SAMPLE 3:
engine load = 50%; G = 70 m3/h; L = 150 L/h.
The experimental results are reported in Table 6 for heavy metals content and in Table 7 for organics content.
The concentrations of some metals such as Fe, Al, Co, Cu, Cr, Mn, Mo, Ni, Pb and Zn significantly increased in the washwater. Organics and PAH levels are mostly below the detection limits and only Aliphatics (C10-C12, C12-C16, C5-C16, C16-C35) and Aromatics C8-C10 were detected. Besides, experiments indicated an increase in the metals concentrations by increasing the engine load and liquid flow rate. In particular,
Figure 8 resumes the trends of the washwater concentration of those pollutants that had a most relevant increase, (more than 2 times the value of the parent seawater) as a function of the load engine. The experiments were presented as the ratio between the concentration of pollutant in the washwater sample and that of the parent seawater (CSAMPLE/CKSW) from Tables 6 and 7.
Figure 8 shows that Zinc, Copper and Aluminum concentration in the washwater increased with the engine load and up to a value between 5 and 10 times that of fresh KSW, while Iron, Cobalt, Manganese, Molybdenum and Lead increased up to 30–100 times. On contrary, a significant increase was observed for chromium which reached a maximum value of almost 4500 times than fresh KSW, while for nickel it reached 650 for the 50% load.
The only two organic species that were higher than KSW were Aliphatics > C16-C35, up to 10 times, and Aromatics > C8-C10, between 3 and 50 times.
Figure 9 compare the discharge concentrations of the heavy metals in the three washwater samples (Tables 6 and 7) with the regulations in Table 8.
-
EU-EQS
Maximum allowed Concentration (MAC) of the Europe Environmental Quality Standards, Directive 2013/39/EC, relating to water quality standards in the European Union (Kjølholt et al. 2012);
-
DE-EQS
Danish Environmental Quality Standards, relating to water quality standards in Denmark (Kjølholt et al. 2012);
-
STR-EQS
Stringent Environmental Quality Standards, relating to more stringent criteria for the inland waters and national territorial waters, established by the Danish Ministry of Environment (Kjølholt et al. 2012);
-
EPA-NRWQC
EPA’s National Recommended Water Quality Criteria for saltwater organisms (United States Environmental Protection Agency, 2011);
-
IT-DL
D.Lgs. 3rd April 2006, No. 152 Environmental Regulations (Italian Official Gazette No. 88 of 14th April 2006—suppl. ord. No. 96) (Italian Official Gazette n. 88 del 14 April 2006—suppl. ord. n. 96, 2006a, 2006b);
-
GE-DL
Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany (Promulgation of the New Version of the Ordinance on Requirements for the Discharge of Waste Water into Waters of 17th June 2004) (Federal Ministry for the Environment Nature Conservation and Nuclear Safety Germany 2004).
It is worth remembering that DL and EQS limits are very different. The DLs refers to the concentration of a pollutant at the discharge point, without any dilution, and is defined by the performances of the best available technologies that are commercialized at the time of introduction of regulation. The EQSs refer to the chemical quality of a natural water body. Indeed, the actual concentration of a chemical in a water body depends both on the anthropogenic emissions and on the natural background and biological and chemical-physical transformations occurring in the same water body. Besides, this concentration strongly depends on the relevance of turbulence and water currents and their effect on the washwater dilution this is extremely important for sea areas.
Despite this, the EQSs have a repercussion on the discharge limit of nearby sources of wastewater emissions: when EQS is exceeded or is close to limit in a certain area, local authorities are entitled to introduce further reduction to the discharge limit of the emission sources affecting the quality of that area. Therefore, while scrubber washwater composition must be compared with the DL limits, their comparison with the EQS and the knowledge of washwater flow rate may be an index of how much the scrubber can contribute to the pollution of a certain sea area.
Figure 9 showed that most of the heavy metals, e.g. Cr, Cu, Ni, Pb and Zn, have quite high concentrations, and failed to comply with the EQS and NRWQC Regulations for water quality standards. Most organics and PAHs (from Table 7) are below the detection limit of the analytic instrument, only Benzene, Naphthalene and Phenanthrene in the first sample (SAMPLE 1) were detected. Their concentration is below the UE-EQS and EPA-NRWQC targets.
The comparison of metals concentration with the IT-DL and GE-DL Regulations, which refer directly to the discharge of washwater from industrial activities or scrubbers, showed that the limits are almost all verified: only Fe and Cr exceed the limits by almost 30%.
Starting from the analysis of fuel samples and their consumption during tests and assuming that all the metals in the fuel are transferred to the water, it is possible to calculate the fraction of heavy metals in the fuel that is transferred to the scrubber washwater as:
where C is the difference between the concentration of a given heavy metal in the washwater (CSAMPLE) and the baseline concentration of the same heavy metals in the raw Kattegat seawater (CKSW). The denominator of Eq. (9) indicated the maximum concentration in the washwater deriving from the fuel: CM (mg/kg) indicates the mass concentration of metals in the fuel, FC (L/h) is fuel consumption for each engine load (see Table 4), ρF = 0.84 kg/L is the MGO fuel density, L (L/h) is the seawater flow rate fed to the scrubber, G (m3/h) is the flue-gas flow rate fed to the scrubber, Geng (m3/h) is the total exhaust gas flow rate produced by the engine (estimated as 144 Nm3/h, on the bases of the engine speed and size). The effect of lubricating oil combustion in the exhaust gases is negligible for this kind of engine. The fractions f for different metals are reported in Table 9.
Table 9 demonstrates that, awkwardly, almost all the metals in the scrubber washwater do not derive from the fuel (corresponding to a ratio f > 1). As suggested by former works (Hufnagl et al. 2005), the authors envisage that the origin of these metals (Fe, Al, Cr, Cu, Mn, Co, Ni, Pb, Zn) can be related to the corrosion of scrubber and pipeline metals. This result makes the choice of the right material of material for FGD systems very important in order to keep emissions of these metals low. Manufacturers usually adopt nickel-based alloys, titanium, or non-metallic materials such as epoxy and composites to prevent or minimize corrosion.
4 Conclusions
This work analyzes the potential reduction of the environmental footprint of ships achievable by the use of an MGO fuel in combination with a scrubber. The tests were carried out in a pilot spray FGD column used to clean the gases deriving from a marine Diesel engine (Volvo Penta 80 kW) operated at different loads with a 0.92% in weight of sulphur in the MGO. The tested scrubbing liquid was a sample Kattegat seawater, used either as is or doped with NaOH.
The rationale for this solution is in its capacity to simultaneously assure a strong reduction of both gas and water emissions. The better quality of the fuel allows reducing the overall emissions of PAHs and heavy metals, while the scrubber reduces sulphur emissions, keeping the concentration of these persistent pollutants well below current washwater limitations, with a limited impact over the environmental quality standard for seawater. Among the metal contaminants, only Fe and Cr exceed the allowed limits by about 30%. It should be noted that for the examined samples the seawater fed flow rate does not offer a significant driving effect on the dilution of the volatile organic and polycyclic aromatic contaminants which are often not detectable with analysis instruments, only Benzene, Naphthalene and Phenanthrene in the first sample were detected but compliant with water quality standards.
As the long-lasting experience of marine scrubbing clearly demonstrated, sulphur emissions control is not a problem, and the pilot-scale scrubber was able to easily cut the SO2 emissions below the SECA and GLOBAL targets. The addition of 100 mg/L of NaOH in seawater determines a further and significant increase in desulphurization efficiencies, reaching the targets required in maritime transport with a water-saving up to 33% compared to the use of pure seawater.
Unfortunately, this process had scarce effects on other gas pollutants (NOx, CO and CO2) because their scarce solubility in water. The experiments also showed that PAHs emissions are within the available water quality standards indicated by the EU and the USA and that corrosion of metallic surfaces of the scrubber and the discharge pipelines is a major source of heavy metals in the scrubber washwater, overwhelming the actual contribution deriving from the transfer of fuel-derived metals to the scrubber effluents. Among the metal contaminants with the highest impact due to corrosion were Iron, Chromium, Nickel and Zinc. Such corrosion problems are probably a relevant contribution to scrubber washwater quality also for Intermediate Fuel Oil (IFO) fueled ships and should be considered also for conventional cooling systems onboard ships, which are commonly used to dilute scrubber effluents before discharge as secondary metals source.
It is worth noticing that a shift to distillate fuels was probably the original intention of the MARPOL VI regulation, but the cost and availability of distillates posed a limit in its use favoring the adoption of desulphurized residual fuels. However, these last kind of fuels do not reduce the actual emissions of other components as ashes or aromatic compounds, limiting the actual environmental benefit of fuel shift.
Indeed, while distillates are the preferred choice for this solution, a benefit can also come from the use of lighter residual marine fuels as RMA10 or RMB30, for which a massive reduction of ashes and carbon micro-residue can be achieved with respect to conventional RMG380/RMK380 fuels.
While the cost of the combined use of lighter fuels and scrubber after-treatment has to be carefully considered and is surely well above those currently adopted to comply with the sulphur limits requirements, it is worth noticing that the proposed solution is able to provide a superior reduction of both the atmospheric and the water emission of ships. Besides, it is worth noticing that both the technologies of engine tuning and modifications required for fuel switching from residual to distillate fuels and for scrubber installation and management are now mature and their implementation in a combined solution is not expected to pose unexpected problems or complexities.
In light of these considerations, the Authors believe that the proposed solution of a combined use of lighter fuels and scrubber after-treatment earns a chance to be considered as a valid option for reducing the environmental footprint of new and existing ships.
Abbreviations
- ASTM:
-
American Society for Testing and Materials
- DE-EQS:
-
Danish Environmental Quality Standards
- EPA:
-
Environmental Protection Agency
- EPA-NRWQ:
-
EPA’s National Recommended Water Quality Criteria for saltwater organisms
- EU-EQS:
-
Europe Environmental Quality Standards
- FGD:
-
Flue-Gas Desulphurization
- GE-DL:
-
German D.Lgs on discharge of waste water into waters
- HFO:
-
Heavy Fuel Oil
- IFO:
-
Intermediate Fuel Oil
- IMO:
-
International Maritime Organization
- ISO:
-
Organization for Standardization
- IT-DL:
-
Italian D.Lgs on Environmental Regulations
- KSW:
-
Kattegat seawater
- KSWOH:
-
Kattegat seawater with NaOH addition
- MAC:
-
Maximum allowed concentration
- MEPC:
-
Marine Environment Protection Committee
- MGO:
-
Marine Gas Oil
- PAH:
-
Polycyclic Aromatic Hydrocarbon
- PPR:
-
Pollution Prevention & Response Sub-Committee
- SECA:
-
Sulphur Emission Control Area
- STR-EQS:
-
Stringent Environmental Quality Standards
- TSS:
-
Total suspended solids
References
Anderson M, Salo K, Hallquist ÅM, Fridell E (2015) Characterization of particles from a marine engine operating at low loads. Atmos Environ 101:65–71
Andreasen A, Mayer S (2007) Use of seawater scrubbing for SO2 removal from marine engine exhaust. Energy Fuels 21:3274–3279. https://doi.org/10.1021/ef700359w
Carnival Corporation & PLC (2019). Compilation and Assessment of Lab Samples from EGCS Washwater Discharge on Carnival ships. DNV-GL, and Carnival Corporation. Available from http://media.corporate-ir.net/media_files/IROL/14/140690/Carnival-DNVGL_Washwater_Analysis_2018.pdf [Accessed on Feb. 2019]
Carotenuto C, Di Natale F, Lancia A (2010) Wet electrostatic scrubbers for the abatement of submicronic particulate. Chem Eng J 165:35–45
Darake S, Hatamipour MS, Rahimi A, Hamzeloui P (2016) SO2 removal by seawater in a spray tower: Experimental study and mathematical modeling. Chem Eng Res Des 109:180–189
Derwent RG, Stevenson DS, Doherty RM, Collins WJ, Sanderson MG, Johnson CE, Cofala J, Mechler R, Amann M, Dentener FJ (2005) The contribution from shipping emissions to air quality and acid deposition in Europe. Ambio 34:54–59
Di Natale F, Carotenuto C (2015) Particulate matter in marine diesel engines exhausts: Emissions and control strategies. Transp Res Part D: Transp Environ 40:166–191
Di Natale F, Carotenuto C, D’Addio L, Jaworek A, Krupa A, Szudyga M, Lancia A (2015) Capture of fine and ultrafine particles in a wet electrostatic scrubber. J Environ Chem Eng 3:349–356. https://doi.org/10.1016/j.jece.2014.11.007
Di Natale F, Carotenuto C, Manna L, Esposito M, La Motta F, D’Addio L, Lancia A (2016) Water electrified sprays for emission control in energy production processes. Int J Heat Technol 34:S597–S602
Di Natale F, La Motta F, Carotenuto C, Tammaro M, Lancia A (2018) Condensational growth assisted Venturi scrubber for soot particles emissions control. Fuel Process Technol 175:77–89
Di Natale F, Carotenuto C, Caserta S, Troiano M, Manna L, Lancia A (2019a) Experimental evidences on the chemi-electro-hydrodynamic absorption of sulphur dioxide in electrified water sprays. Chem Eng Res Des 146:249–262
Di Natale F, Carotenuto C, Parisi A, Lancia A (2019b). Enhancing the efficiency of a seawater-based FGD process using induction charging sprays. Proceedings of the 42nd ASICI, Ravenna, 38–43
Di Natale F, Carotenuto C, Sippula O, Gregory D (2020a). A bottom-up evaluation of the contribution of marine traffic on particle concentrations in a Mediterranean coastal area: Effects of fuel quality and exhaust gas cleaning systems. Atmosphere, Special Issue "Air Quality and Sustainable Development of Urban Agglomerations in the Mediterranean Area: Science, Technology and Policies, in press
Di Natale F, Carotenuto C, Parisi A, Lancia A (2020b) Absorption of sulphur dioxide by electrosprayed droplets. The Canadian Journal of Chemical Engineering 98:1989–1997
Dulière V, Baetens K, Lacroix G (2020) Potential impact of wash water effluents from scrubbers on water acidification in the southern North Sea. Operation Directorate Natural Environment Technical Report No. 21–04–2020. Royal Belgian Institute of Natural Sciences, Brussels Belgium
Endres S, Maes F, Hopkins F, Houghton K, Mårtensson EM, Oeffner J, Quack B, Singh P, Turner D (2018) A new perspective at the ship-air-sea-interface: The environmental impacts of exhaust gas scrubber discharge. Front Mar Sci 5:1–13
Eyring V, Köhler HW, Lauer A, Lemper B (2005) Emissions from international shipping: 2. Impact of future technologies on scenarios until 2050. J Geophys Res D: Atmos 110:183–200
Federal Ministry for the Environment Nature Conservation and Nuclear Safety Germany (2004). Promulgation of the New Version of the Ordinance on Requirements for the Discharge of Waste Water into Waters (Waste Water Ordinance - AbwV). Available from https://www.bmu.de/fileadmin/bmu-import/files/pdfs/allgemein/application/pdf/wastewater_ordinance.pdf [Accessed on June 17 2004]
Flagiello D, Di Natale F, Erto A, Lancia A (2017). Marine diesel engine flue gas desulphurization by seawater scrubbing in a structured packing absorption column. Proceedings of the 40th ASICI, Rome, 1–6
Flagiello D, Erto A, Lancia A, Di Natale F (2018a) Experimental and modelling analysis of seawater scrubbers for sulphur dioxide removal from flue-gas. Fuel 214:254–263. https://doi.org/10.1016/j.fuel.2017.10.098
Flagiello D, Di Natale F, Carotenuto C, Erto A, Lancia A (2018b) Seawater desulphurization of simulated flue gas in spray and packed columns: An experimental and modelling comparison. Chem Eng Trans 69:799–804
Flagiello D, Lancia A, Erto A, Di Natale F (2019a). Desulphurization of combustion flue-gases by Wet Oxidation Scrubbing (WOS). Proceedings of the 42th ASICI, Ravenna, 5–10
Flagiello D, Parisi A, Lancia A, Carotenuto C, Erto A, Di Natale F (2019b) Seawater desulphurization scrubbing in spray and packed columns for a 4.35 MW marine diesel engine. Chem Eng Res Des 148:56–67. https://doi.org/10.1016/j.cherd.2019.05.057
Flagiello D (2020). Enhanced seawater scrubbing for fue-gas cleaning. PhD thesis, University of Naples, Federico II
Flagiello D, Di Natale F, Lancia A, Erto A (2020a) Characterization of mass transfer coefficients and pressure drops for packed towers with Mellapak 250.X. Chem Eng Res Des 161:340–356. https://doi.org/10.1016/j.cherd.2020.06.031
Flagiello D, Di Natale F, Erto A, Lancia A (2020b) Wet oxidation scrubbing (WOS) for flue-gas desulphurization using sodium chlorite seawater solutions. Fuel 277:118055. https://doi.org/10.1016/j.fuel.2020.118055
Flagiello D, Erto A, Lancia A, Di Natale F (2020c). Dataset of wet desulphurization scrubbing in a column packed with Mellapak 250.X. Data in Brief, 33: 106383https://doi.org/10.1016/j.dib.2020.106383
Gregory D, Confuorto N (2012). A practical guide to exhaust gas cleaning systems for the maritime industry. Exhaust Gas Cleaning Systems Association (EGCSA), London, UK, Technical Report No. 01-2012
Ha TH, Nishida O, Fujita H, Wataru H (2010) Enhancement of diesel particulate matter collection in an electrostatic water-spraying scrubber. J Mar Sci Technol 15:271–279
Hufnagl M, Liebzeit G, Behrends B (2005) Effects of Seawater Scrubbing Technical Report No. 03–2020. Research Centre Terramare, Schleusenstrass, Germany
Iliuta I, Larachi F (2001) Mechanistic model for structured-packing-containing columns: Irrigated pressure drop, liquid holdup, and packing fractional wetted area. Ind Eng Chem Res 40:5140–5146
Iliuta I, Larachi F (2019) Modeling and simulations of NOx and SO2 seawater scrubbing in packed-bed columns for marine applications. Catalysts 9:489
Iliuta I, Iliuta MC (2019) Modeling of SO2 seawater scrubbing in countercurrent packed-bed columns with high performance packings. Sep Purif Technol 226:162–180. https://doi.org/10.1016/j.seppur.2019.05.078
International Maritime Organization (2019). MEPC 74/INF.10: pollution prevention and response scrubber environmental impact literature review submitted by Panama
International Maritime Organization (2006). MEPC 55/4/5: prevention of air pollution from ships - washwater criteria guidelines for exhaust gas cleaning systems-SOx (EGCS-SOx) Units
International Maritime Organization (2008). RESOLUTION MEPC.176(58): amendments to the Annex of the Protocol of 1997 to amend the International Convention for the Prevention of pollution from the ship, 1973, as modified by the Protocol of 1978 relating Thereto (Revised MARPOL Annex VI)
International Maritime Organization (2015) Guidelines for exhaust gas cleaning systems (MEPC.259(68))
Italian Official Gazette n. 88 - suppl. ord. n. 96. (2006a). D.Lgs. 152/06 - Norme in materia ambientale. Available from https://www.gazzettaufficiale.it/eli/gu/2006/04/14/88/sg/pdf [Accessed on April 14 2006]
Italian Official Gazette n. 88 - suppl. ord. n. 96. (2006b). D. Lgs 152/06 (Parte terza, Allegato 5, Tabella 3) - Valori limiti di emissioni in acque superficiali e in fognatura. Available from http://bresciacaffaro.it/images/documenti_da_scaricare/inquinamento/VALORI-LIMITI-DI-EMISSIONE-IN-ACQUE-SUPERFICIALI-E-IN-FOGNATURA.PDF [Accessed on April 14 2006]
Kjølholt J, Aakre S, Jürgensen C, Lauridsen J (2012) Assessment of possible impacts of scrubber water discharges on the marine environment. Danish EPA Technical Report No. 1431. Danish Ministry of the Environment, København
Kuang M, Wang J, Hu X, Yang G (2020) Seawater/Seawater cascade-scrubbing desulfurization performance for exhaust gas of a 162-kW marine diesel engine. Journal of Environmental Engineering (united States) 146:1–11
Lange B, Markus T (2015) Impacts of Scrubbers on the environmental situation in ports and coastal waters. FEA Technical Report No. (UBA-FB) 002015/E. Federal Environment Agency, Germany
Lee K, Tong LT, Millero FJ, Sabine CL, Dickson AG, Goyet C, Park GH, Wanninkhof R, Feely RA, Key RM (2006) Global relationships of total alkalinity with salinity and temperature in surface waters of the world’s oceans. Geophys Res Lett 33:1–5
Lehtoranta K, Aakko-Saksa P, Murtonen T, Vesala H, Ntziachristos L, Rönkkö T, Karjalainen P, Kuittinen N, Timonen H (2019) Particulate mass and nonvolatile particle number emissions from marine engines using low-sulfur fuels, natural gas, or scrubbers. Environ Sci Technol 53:3315–3322
Liu JJ, Wang F, Liu H, Wei YB, Li H, Yue J, Que J, Degenhardt L, Lappin J, Lu L, Bao Y, Wang J (2019) Association of ambient fine particulate matter with increased emergency ambulance dispatches for psychiatric emergencies: a time-series analysis. The Lancet 394:S7. https://doi.org/10.1016/S0140-6736(19)32343-8
Oeder S, Kanashova T, Sippula O, Sapcariu SC, Streibel T, Arteaga-Salas JM, Passig J, Dilger M, Paur H, Schalger C, Mülhopt S, Diabaté S, Weiss C, Stengel B, Rabe R, Harndorf H, Torvela T, Jokineimi JK, Hirvonen M, Schmidt-Weber C, Tradl-Hoffmann C, BéruBé K, Wlodarczyk AJ, Prytherch Z, Michalke B, Krebs T, Prévôt A, Kelbg M, Tiggesbäumker J, Karg E, Jakobi G, Scholtes S, Kreis J, Lintelmann J, Matuschek G, Sklorz M, Klingbeil S, Orasche J, Richthammer P, Müller L, Elsasser M, Reda A, Gröger T, Weggler B, Schwemer T, Czech H, Rüger C, Abbaszade G, Radischat R, Hiller K, Buters J, Dittmar G, Zimmerman R (2015) Particulate matter from both heavy fuel oil and diesel fuel shipping emissions show strong biological effects on human lung cells at realistic and comparable in vitro exposure conditions. PLoS ONE 10:1–17
Reis H, Reis C, Sharip A, Reis W, Zhao Y, Sinclair R, Beeson L (2018) Diesel exhaust exposure, its multi-system effects, and the effect of new technology diesel exhaust. Environ Int 114:252–265
Rodríguez-Sevilla J, Álvarez M, Díaz MC, Marrero MC (2004) Absorption equilibria of dilute SO2 in seawater. J Chem Eng Data 49:1710–1716
Schmid O, Möller W, Semmler-Behnke M, Ferron GA, Karg E, Lipka J, Schulz H, Kreyling WG, Stoeger T (2009) Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers 14:67–73
Schultes M, Brauer J, Chen P, Doong S (2018) Marinization of mass transfer columns for FLNG applications. Chem Eng Trans 69:301–306
Tang XJ, Li T, Yu H, Zhu YM (2014) Prediction model for desulphurization efficiency of onboard magnesium-base seawater scrubber. Ocean Eng 76:98–104. https://doi.org/10.1016/j.oceaneng.2013.11.009
Teuchies J, Cox TJS, Van Itterbeeck K, Meysman FJR, Blust R (2020) The impact of scrubber discharge on the water quality in estuaries and ports. Environ Sci Eur 32:103
Turner DR, Hassellöv IM, Ytreberg E, Rutgersson A (2017) Shipping and the environment: Smokestack emissions, scrubbers and unregulated oceanic consequences. Elementa 5:45
Turner DR, Edman M, Gallego-Urrea JA, Claremar B, Hassellöv IM, Omstedt A, Rutgersson A (2018) The potential future contribution of shipping to acidification of the Baltic Sea. Ambio 47:368–378
Wang J, Kuang M, Yang G, Hu X, Xu Y, Fan P (2019) Desulfurization performance comparison of a 162-kW marine diesel engine’s exhaust gas based on two kinds of alkaline liquid scrubbing models. Asia-Pac J Chem Eng 14:1–13
Wichmann HE (2007) Diesel exhaust particles. Inhalation Toxicol 19:241–244
Winnes H, Fridell E, Moldanová J (2020) Effects of marine exhaust gas scrubbers on gas and particle emissions. Journal of Marine Science and Engineering 8:299
Ytreberg E, Hassellöv IM, Nylund AT, Hedblom M, Al-Handal AY, Wulff A (2019) Effects of scrubber washwater discharge on microplankton in the Baltic Sea. Mar Pollut Bull 145:316–324. https://doi.org/10.1016/j.marpolbul.2019.05.023
Zetterdahl M, Moldanová J, Pei X, Pathak RK, Demirdjian B (2016) Impact of the 0.1% fuel sulfur content limit in SECA on particle and gaseous emissions from marine vessels. Atmos Environ 145:338–345
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Article Highlights
• A new model to contain emission footprint of maritime shipping is proposed.
• A pilot-scale open-loop scrubber was tested to treat a Diesel engine exhausted gas.
• SO2 scrubber emissions from MGO fuel were complying with IMO limits.
• 25%-33% of water-savings were achieved by adding NaOH in pure seawater.
• Most of the pollutants in the wash water complied with the Water Quality Standards.
• Heavy metals emissions in wash water were related to corrosion of the set-up alloys
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flagiello, D., Esposito, M., Di Natale, F. et al. A Novel Approach to Reduce the Environmental Footprint of Maritime Shipping. J. Marine. Sci. Appl. 20, 229–247 (2021). https://doi.org/10.1007/s11804-021-00213-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11804-021-00213-2