Abstract
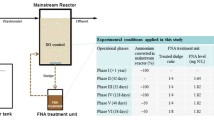
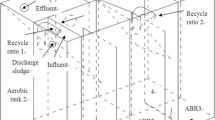
The effects of hydraulic retention time (HRT) on the nitrification activities and population dynamics of a conventional activated sludge system fed with synthetic inorganic wastewater were investigated over a period of 260 days. When the HRT was gradually decreased from 30 to 5 h, the specific ammonium-oxidizing rates (SAOR) varied between 0.32 and 0.45 kg NH +4 -N (kg mixed liquor suspended solids (MLSS)·d)−1, and the specific nitrate-forming rates (SNFR) increased from 0.11 to 0.50 kg NO −3 -N (kg MLSS·d)−1, showing that the decrease in HRT led to a significant increase in the nitrite oxidation activity. According to fluorescence in situ hybridization (FISH) analysis results, the proportion of ammonia-oxidizing bacteria (AOBs) among the total bacteria decreased from 33% to 15% with the decrease in HRT, whereas the fraction of nitrite-oxidizing bacteria (NOBs), particularly the fast-growing Nitrobacter sp., increased significantly (from 4% to 15% for NOBs and from 1.5% to 10.6% for Nitrobacter sp.) with the decrease in HRT, which was in accordance with the changes in SNFR. A short HRT favored the relative growth of NOBs, particularly the fast-growing Nitrobacter sp., in the conventional activated sludge system.
Similar content being viewed by others
References
Campos J L, Garrido-Fernandez J M, Mendez R, Lema J M. Nitrification at high ammonia loading rates in an activated sludge unit. Bioresource Technology, 1999, 68(2): 141–148
Carrera J, Baeza J A, Vicent T, Lafuente J. Biological nitrogen removal of high-strength ammonium industrial wastewater with two-sludge system. Water Research, 2003, 37(17): 4211–4221
Geets J, de Cooman M, Wittebolle L, Heylen K, Vanparys B, de Vos P, Verstraete W, Boon N. Real-time PCR assay for the simultaneous quantification of nitrifying and denitrifying bacteria in activated sludge. Applied Microbiology and Biotechnology, 2007, 75(1): 211–221
Okabe S, Satoh H, Watanabe Y. In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Applied and Environmental Microbiology, 1999, 65(7): 3182–3191
Satoh H, Yamakawa T, Kindaichi T, Ito T, Okabe S. Community structures and activities of nitrifying and denitrifying bacteria in industrial wastewater-treating biofilms. Biotechnology and Bioengineering, 2006, 94(4): 762–772
Siripong S, Rittmann B E. Diversity study of nitrifying bacteria in full-scale municipal wastewater treatment plants. Water Research, 2007, 41(5): 1110–1120
Dionisi H M, Layton A C, Robinson K G, Brown J R, Gregory I R, Parker J J, Sayler G S. Quantification of Nitrosomonas oligotropha and Nitrospira spp. using competitive polymerase chain reaction in bench-scale wastewater treatment reactors operating at different solids retention times. Water Environment Research, 2002, 74(5): 462–469
Grady C P, Daigger G T, Lim H C. Biological Wastewater Treatment. New York: Marcel Dekker Inc, 1999
Qin Y Y, Zhang X W, Ren H Q, Li D T, Yang H. Population dynamics of ammonia-oxidizing bacteria in an aerated submerged biofilm reactor for micropolluted raw water pretreatment. Applied Microbiology and Biotechnology, 2008, 79(1): 135–145
Satoh H, Okabe S, Yamaguchi Y, Watanabe Y. Evaluation of the impact of bioaugmentation and biostimulation by in situ hybridization and microelectrode. Water Research, 2003, 37(9): 2206–2216
Park H D, Noguera D R. Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Research, 2004, 38(14–15): 3275–3286
Horz H P, Rotthauwe J H, Lukow T, Liesack W. Identification of major subgroups of ammonia-oxidizing bacteria in environmental samples by T-RFLP analysis of amoA PCR products. Journal of Microbiological Methods, 2000, 39(3): 197–204
You S J, Hsu C L, Chuang S H, Ouyang C F. Nitrification efficiency and nitrifying bacteria abundance in combined AS-RBC and A2O systems. Water Research, 2003, 37(10): 2281–2290
Mobarry B K, Wagner M, Urbain V, Rittmann B E, Stahl D A. Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Applied and Environmental Microbiology, 1996, 62(6): 2156–2162
Brandt K K, Hesselsøe M, Roslev P, Henriksen K, Sørensen J. Toxic effects of linear alkylbenzene sulfonate on metabolic activity, growth rate, and microcolony formation of Nitrosomonas and Nitrosospira strains. Applied and Environmental Microbiology, 2001, 67(6): 2489–2498
Schramm A. Beer de D, Heuvel van den J C, Ottengraf S, Amann R. Microscal distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Applied and Environmental Microbiology, 1999, 65(8): 3690–3696
Rowan A K, Snape J R, Fearnside D, Barer M R, Curtis T P, Head I M. Composition and diversity of ammonia-oxidising bacterial communities in wastewater treatment reactors of different design treating identical wastewater. FEMS Microbiology Ecology, 2003, 43(2): 195–206
Yu T, Qi R, Li D, Zhang Y, Yang M. Nitrifier characteristics in submerged membrane bioreactors under different sludge retention times. Water Research, 2010, 44(9): 2823–2830
Nogueira R, Melo L F, Purkhold U, Wuertz S, Wagner M. Nitrifying and heterotrophic population dynamics in biofilm reactors: effects of hydraulic retention time and the presence of organic carbon. Water Research, 2002, 36(2): 469–481
Li H Y, Zhang Y, Gao F, Yu T, Yang M. Effects of hydraulic retention time (HRT) on nitrification performance and microbial community of conventional activated sludge (CAS). Environmental Science, 2006, 27(9): 1862–1865 (in Chinese)
Kurisu F, Satoh H, Mino T, Matsuo T. Microbial community analysis of thermophilic contact oxidation process by using ribosomal RNA approaches and the quinone profile method. Water Research, 2002, 36(2): 429–438
Gao M, Yang M, Li H, Yang Q, Zhang Y. Comparison between a submerged membrane bioreactor and a conventional activated sludge system on treating ammonia-bearing inorganic wastewater. Journal of Biotechnology, 2004, 108(3): 265–269
Environmental Protection Bureau. The Standard Methods of Water and Wastewater Monitoring and Analysis of China, 4th ed. Beijing: China Environmental Science Press, 2002
Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiological Reviews, 1995, 59(1): 143–169
Li H Y, Yang M, Zhang Y, Yu T, Kamagata Y. Nitrification performance and microbial community dynamics in a submerged membrane bioreactor with complete sludge retention. Journal of Biotechnology, 2006, 123(1): 60–70
Hesselose M, Brandt K K, Sorensen J. Quantification of ammonia oxidizing bacteria in soil using microcolony technique combined with fluorescence in situ hybridization (MCFU-FISH). FEMS Microbiology Ecology, 2001, 38(2–3): 87–95
Persson F, Wik T, Sörensson F, Hermansso M. Distribution and activity of ammonia oxidizing bacteria in a large full-scale trickling filter. Water Research, 2002, 36(6): 1439–1448
Purkhold U, Pommerening-Röser A, Juretschko S, Schmid M C, Koops H P, Wagner M. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Applied and Environmental Microbiology, 2000, 66(12): 5368–5382
Luxmy B S, Nakajima F, Yamamoto K. Analysis of bacterial community in membrane-separation bioreactors by fluorescent in situ hybridization (FISH) and denaturing gradient gel electrophoresis (DGGE) techniques. Water Science and Technology, 2000, 41(10–11): 259–268
Wagner M, Rath G, Koops H-P, Flood J, Amann R. In situ analysis of nitrifying bacteria in sewage treatment plants. Water Science and Technology, 1996, 34(1–2): 237–244
Wang X, Wen X, Criddle C, Wells G, Zhang J, Zhao Y. Community analysis of ammonia-oxidizing bacteria in activated sludge of eight wastewater treatment systems. Journal of Environmental Sciences (China), 2010, 22(4): 627–634
Andrews JH, Harris RF. γ- and k- Selection and microbial ecology. Advances in Microbial Ecology, 1986, 9: 99–147
Schramm A, De Beer D, Gieseke A, Amann R. Microenvironments and distribution of nitrifying bacteria in a membrane-bound biofilm. Environmental Microbiology, 2000, 2(6): 680–686
Stehr G, Bottcher B, Dittberner P, Rath G, Koops H P. The ammonia-oxidizing nitrifying population of the River Elbe estuary. FEMS Microbiology Ecology, 1995, 17(3): 177–186
Hibiya K, Terada A, Tsuneda S, Hirata A. Simultaneous nitrification and denitrification by controlling vertical and horizontal microenvironment in a membrane-aerated biofilm reactor. Journal of Biotechnology, 2003, 100(1): 23–32
Copp J B, Murphy K. Estimation of the active nitrifying biomass in activated sludge. Water Research, 1995, 29(8): 1855–1862
Daims H, Ramsing N B, Schleifer K H, Wagner M. Cultivation-independent, semiautomatic determination of absolute bacterial cell numbers in environmental samples by fluorescence in situ hybridization. Applied and Environmental Microbiology, 2001, 67(12): 5810–5818
Hagopian D S, Riley J G. A closer look at the bacteriology of nitrification. Aquacultural Engineering, 1998, 18(4): 223–244
Koop H-P, Pommerening-Roser A. Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiology Ecology, 2001, 37(1): 1–9
Manser R. Population dynamics and kinetics of nitrifying bacteria in membrane and conventional activated sludge plants. Dissertation for the Doctoral Degree. Swiss Federal Institute of Technology, Zurich, 2005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Zhang, Y., Yang, M. et al. Effects of hydraulic retention time on nitrification activities and population dynamics of a conventional activated sludge system. Front. Environ. Sci. Eng. 7, 43–48 (2013). https://doi.org/10.1007/s11783-012-0397-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-012-0397-8