Abstract
Purpose
To systematically review and meta-analyse the efficacy of exercise interventions delivered before and/or during taxane-containing chemotherapy regimens on chemotherapy-induced peripheral neuropathy (CIPN), fatigue, and health-related quality of life (HR-QoL), in women with breast cancer.
Methods
Seven electronic databases were systematically searched for randomised controlled trials (RCTs) reporting on the effects of exercise interventions in women with breast cancer receiving taxane-containing chemotherapeutic treatment. Meta-analyses evaluated the effects of exercise on CIPN symptoms, fatigue, and HR-QoL.
Results
Ten trials involving exercise interventions ranging between 2 and 12 months were included. The combined results of four RCTs consisting of 171 participants showed a reduction in CIPN symptoms following exercise compared with usual care (standardised mean difference − 0.71, 95% CI − 1.24 to − 0.17, p = 0.012; moderate-quality evidence, I2 = 76.9%). Pooled results from six RCTs with 609 participants showed that exercise interventions before and/or during taxane-containing chemotherapy regimens improved HR-QoL (SMD 0.42, 95% CI 0.07 to 0.76, p = 0.03; moderate-quality evidence, I2 = 49.6%). There was no evidence of an effect of exercise on fatigue (− 0.39, 95% CI − 0.95 to 0.18, p = 0.15; very low-quality evidence, I2 = 90.1%).
Conclusions
This systematic review found reduced levels of CIPN symptoms and an improvement in HR-QoL in women with breast cancer who exercised before and/or during taxane-based chemotherapy versus usual care controls.
Implications for Cancer Survivors
This evidence supports the role of exercise as an adjunctive treatment for attenuating the adverse effects of taxane-containing chemotherapy on CIPN symptoms and HR-QoL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to advances in screening, early detection, and treatment, there are more people living with and beyond a breast cancer diagnosis than ever [1]. Major advancements in the treatment of cancer involve the use of modern chemotherapy, including cytotoxic agents such as platinum compounds and taxanes. However, their long- and short-term side effects can negatively impact a patients’ health-related quality of life (HR-QoL), during and after chemotherapy [2, 3]. This has prompted the need to investigate strategies to reduce the side-effects of treatment and improve HR-QoL.
Taxanes (e.g. paclitaxel, docetaxel) are among the most active cytotoxic chemotherapy drugs available for breast cancer [4]. The National Institute for Health and Clinical Excellence (NICE) recommends that taxanes are combined with anthracyclines for the treatment of invasive breast cancer, noting that the benefits of adding taxanes into a treatment regimen include reducing the risk of breast cancer recurrence and increasing survival rate [5]. However, taxanes also affect the structure and function of peripheral sensory, motor, and autonomic neurons [6], with the resultant impact often manifesting as chemotherapy-induced peripheral neuropathy (CIPN). Symptoms of CIPN include hand and foot numbness, paraesthesia, pain, and impairments to balance, gait, and posture [7]. Incidence rates of CIPN can range from 11 to 87%, depending on the specific drug and treatment regimen [8]. The burden of CIPN commonly results in dose-reduction and premature termination of treatment [9, 10]. These often severe symptoms can manifest alongside further debilitating side effects that can influence patient quality of life. Studies have found that 100% of patients receiving taxanes experienced fatigue [11, 12], with fatigue being the symptom experienced most severely [11]. Both cancer-related fatigue and CIPN symptoms are associated with reduced HR-QoL [13, 14].
Pharmacological interventions aimed at preventing CIPN have very limited evidence of efficacy [15, 16]. Duloxetine is the only currently recommended drug for paclitaxel-induced CIPN [17]; however, duloxetine use must be closely monitored by a physician and is associated with side effects (nausea, insomnia, and dizziness) [18]. Furthermore, a recent systematic review found duloxetine and placebo to be similar in efficacy [19]. Thus, an increasing body of research is being conducted into the impact of non-pharmacological interventions on CIPN and other common symptoms (including cancer-related fatigue) across a range of cancers. Exercise has been shown to enhance the expression of neurotrophic factors [20], reduce inflammation [21], and regulate mitochondrial dysfunction implicated in the development of CIPN [22,23,24]. A systematic review found that exercise during a variety of chemotherapy regimens improves CIPN symptoms and postural control [25], and exercise performed peri-chemotherapy, including after, reduces CIPN symptoms [26] and neuropathic pain [27]. Exercise during chemotherapy has also been shown to improve HR-QoL [25, 27], and exercise during adjuvant treatment for breast cancer reduces fatigue and cancer site-specific quality of life [28].
Although current data suggest that exercise has potential to alleviate symptoms of CIPN, available evidence syntheses have included exercise interventions prescribed to participants at any time point throughout their chemotherapy. Interventions that are given to participants post-chemotherapy could be tracking a natural easing of CIPN symptoms [29] and improvement of HR-QoL [30], therefore aiding rehabilitation as opposed to modulating CIPN symptom severity (and potentially, the underpinning neuropathology). Furthermore, previous reviews of exercise before and/or during chemotherapy have not investigated participants only receiving taxane-containing chemotherapy regimens. The mechanisms and symptoms of CIPN vary greatly across drug types, each potentially requiring unique management strategies [31, 32]. Moreover, CIPN can have important clinical implications for those receiving taxanes; 17% of those receiving taxanes require a dose reduction due to symptoms of CIPN specifically [10]. Taxane dose reduction is of significant concern as tumour control is associated with increased dose intensity [33]. Additionally, although CIPN severity often decreases gradually after treatment, symptoms frequently persist for at least 6 months after treatment cessation [14]. Therefore, identifying exercise as a potential adjunctive treatment to reduce the severity and/or risk of CIPN symptoms could benefit the immediate and long-term outcomes of treatment and reduce the impact of treatment well beyond chemotherapy termination. Thus, we systematically reviewed and meta-analysed the effect of exercise interventions before and/or during taxane-containing chemotherapy regimens on CIPN, fatigue, and HR-QoL in women undergoing breast cancer treatment.
Methods
This systematic review was prospectively registered in the PROSPERO prospective register of systematic reviews (CRD42021272036) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [34]. There were some minor deviations from the study protocol, which are outlined and justified in Supplementary Material 1.
Search strategy
An electronic search of PubMed, EMBASE, Cochrane Central, SPORTDiscus, CINAHL, ClinicalTrials.gov, and ISRTCN was run independently by two authors (RB-S, JT) from inception to 15th September 2022. Within the search, three key concepts were used, specifically breast cancer, exercise, and taxane-containing chemotherapy regimes in addition to their synonyms and controlled vocabulary (e.g. Medical Subject Headings). The search strategy used for each database is presented in Supplementary Material 2.
Eligibility criteria
To be included in this review, studies had to be randomised control trials (RCTs) that recruited women with a breast cancer diagnosis, receiving any chemotherapy regimen containing taxanes. Participants had to have been ≥ 18 years old and randomised to either receive an exercise intervention before and/or during treatment or to usual care. We operationalised the control group as a group of participants that received standard care only or standard care plus the recommendation to follow general physical activity and/or healthy eating guidelines but did not receive the intended study intervention. Full-text articles in any language were eligible. It was required that outcomes included at least one of the following symptoms: CIPN, fatigue, or HR-QoL. Reviews, magazines, surveys, opinion pieces, commentaries, books, periodicals, editorials, conference abstracts, and case studies were excluded as were quasi-experimental, observational, and cross-over studies.
The exercise intervention must have been performed before and/or during the taxane-containing chemotherapy regimen. For the purpose of this review, exercise was defined as a subset of physical activity that is planned, structured, and repetitive and purposefully undertaken to improve health or fitness [35]. Interventions must have included a minimum of two exercise sessions and could have been aerobic, resistance, physical therapy, home-based, facility-based, unsupervised, or supervised. Exercise interventions could also have been given alongside a nutritional intervention. Included studies were required to provide data for a baseline assessment before any chemotherapy or exercise and a follow-up assessment immediately after chemotherapy termination. If the intervention continued beyond the end of chemotherapy, there must be data for included measures from an assessment point at the end of chemotherapy that could be compared to baseline.
Outcomes
The outcomes included in this review were symptoms of CIPN, fatigue, and HR-QoL. The primary outcome was the difference in CIPN symptoms between intervention and usual care groups. For a CIPN outcome to be included in the systematic review, it must have been either generated from a CIPN-specific measure (e.g. EORTC QLQ-30 CIPN20) or be a previously reported, and specifically tested, CIPN symptom (e.g. balance). If not derived from a dedicated questionnaire, other measures of symptoms must have been explicitly assessing CIPN. Eligible symptoms included positive motor and sensory symptoms (hyperalgesia, allodynia, pain, dysesthesia, paraesthesia, muscle cramps, muscle aches) and negative motor and sensory symptoms (numbness, impaired fine motor skills, disturbance of vibratory and proprioceptive sensations, including balance and falls) [16, 36,37,38,39,40]. Secondary outcomes were differences in fatigue and HR-QoL. For a fatigue or HR-QoL outcome to be included in the systematic review, it must have been either a patient- or physician-reported index score, or subscale, of a fatigue or HR-QoL-specific assessment tool. The difference between baseline and follow-up scores from the intervention and usual care groups were compared for all outcomes.
Study selection
After the completion of the literature searches, studies were collated into an Excel spreadsheet, and duplicates were removed by one reviewer (RB-S). Two reviewers (RB-S, JT) then independently screened titles and abstracts for eligibility. Full texts were then obtained for all studies that needed further assessment for eligibility. The same two reviewers then independently examined each full-text manuscript. Any disagreements were resolved via consensus meetings and consultation with a third author (STO).
Data extraction
Data extraction was completed in duplicate by two reviewers (RB-S and JT) using a piloted data extraction form. The data items that were extracted from the included studies were authors, title, year of publication, study design, sample size, participant characteristics (e.g. age), treatment details, type and characteristics of the intervention and usual care groups, outcome measures, baseline and follow-up data (mean and SD), and rates of adherence to intervention. In the case of missing data, corresponding authors were contacted on at least two occasions within a 1-month period. If SDs were not reported, we collected other relevant data that could be converted to SDs, such as 95% confidence intervals (CIs) or p-values.
Risk of bias
The Cochrane risk of bias tool for randomised trials (RoB2) [41] was used to assess the risk of bias for each study outcome of interest within each study. Judgements were made independently by two authors (RB-S, JT), with any disagreements being resolved by discussion and consensus. Availability of data was considered sufficient when there was data for 85% of randomised participants. This was based on the trial context potentially resulting in higher rates of dropout when compared to trials of pharmaceutical interventions.
When a meta-analysis included 10 or more effect sizes, the risk of bias due to missing results in a synthesis was explored with Egger’s test of the intercept [42] and by visually inspecting a funnel plot of the effect estimates plotted against their corresponding sampling variance.
Quality of evidence
The quality of evidence found was assessed using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach [43]. Risk of bias, inconsistency of results, indirectness of evidence, imprecision of results, and publication bias were assessed for each individual outcome. The evidence was downgraded by one level if judged to have a serious limitation or by two levels if judged to have a very serious limitation. GRADE assessments were performed by two independent authors (RB-S, JT), and conflicts were resolved through consensus.
Statistical analysis
A meta-analysis of standardised mean differences (SMDs) between exercise and usual care groups was performed where two or more trials reported the same outcome. SMDs were calculated as the between-group difference in change scores (or difference in post-intervention scores if change scores were not available) divided by the pooled SD at baseline [44]. If SDs were not presented in the study, the SD was estimated from the reported standard error, 95% CI, or p-value. Qualitative descriptors used to interpret the strength of the SMDs were based on Cohen’s criteria [45] ( ±): trivial (< 0.2), small (0.2 to 0.49), moderate (0.5 to 0.79), and large (≥ 0.8).
Meta-analyses were performed with a random effect model using the inverse-variance method, where the weight of each study is the inverse of the variance of the effect estimate. The random effect model was chosen to incorporate potential heterogeneity. CIs and test statistics were calculated via a t-distribution using the Hartung-Knapp-Sidik-Jonkman (HKSJ) approach [46]. When a meta-analysis included more than one outcome measure from the same study, effect estimates were nested within studies using a multi-level structure to account for correlated effects [47].
A χ2 test was used to assess heterogeneity, with p < 0.1 indicating a significant degree of heterogeneity. The I2 statistic was then used to assess the percentage of variability in effect estimates due to heterogeneity rather than sampling error. The I2 thresholds used were in line with Cochrane guidelines; 0–40% (‘might not be important’), 30–60% (‘may represent moderate heterogeneity’), 50–90% (‘may represent substantial heterogeneity’), and 75–100% (‘considerable heterogeneity’) [48]. When a meta-analysis included 10 or more effect estimates and there was evidence of at least moderate heterogeneity, we performed meta-regressions to explore sources of heterogeneity, specifically the impact of the covariates (1) whether the outcome was objectively or subjectively measured and (2) whether the outcome assessed sensory symptoms or motor/autonomic symptoms.
We conducted sensitivity analyses on the main meta-analysis models to explore whether decisions made in the review process influenced the overall findings. Sensitivity analyses involved (1) computing test statistics and 95% CIs based on a normal (z) distribution rather than a t-distribution; (2) using imputed change-from-baseline SD to calculate effect estimates, rather than the SD at baseline; and (3) excluding studies where participants received chemoradiotherapy. Change-from-baseline SD was imputed using a correlation coefficient of 0.7 [49, 50]. We then performed a leave-one-out sensitivity analysis to assess whether removing an individual effect estimate from a meta-analysis influenced the model parameters.
Statistical analyses were conducted using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at p < 0.05. Data are presented as effect estimates with their corresponding 95% CI. The search results, dataset, and statistical analysis are available on Open Science Framework (OSF) repository (https://osf.io/bg896/?view_only=d70613ca97fb41689207a1a240b090df).
Results
Study selection
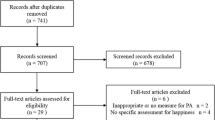
The search of included databases generated 3673 results, of which 1415 were duplicates, and 2258 were screened by title and abstract. Full-text screening of 169 articles found 10 trials that met the eligibility criteria. One of the trials has data reported in two different papers [51, 52]. A summary of the study selection process is presented in Fig. 1.
‘Near misses’
A total of 32 studies were judged to meet many, but not all, of the eligibility criteria (i.e. ‘near misses’). The primary exclusion reason shared by all ‘near miss’ studies was the inclusion of participants receiving a mixture of chemotherapy types and the lack of a distinct dataset for those participants receiving taxane-containing chemotherapies. A full list of these studies and justifications for exclusion are presented in Supplementary Material 3.
Study characteristics
A summary of general study characteristics is presented in Table 1. A total of 896 participants were included in this review, of which 171 were included in the primary CIPN meta-analysis, 737 included in the fatigue meta-analysis, and 609 included in the HR-QoL meta-analysis. Eight of the studies compared an exercise group to a usual care group [53,54,55,56,57,58,59,60], while two studies compared an immediate exercise group to a delayed exercise group that received usual care during the study [51, 52, 61]. One study included patients who had potentially received both chemotherapy and radiotherapy between baseline and post-intervention assessment [61]. Two of the studies were undertaken in Canada [51,52,53], one in Turkey [58], two in the USA [55, 59], one in Germany [60], and four in France [54, 56, 57, 61].
There was a wide variety of exercise interventions included in this review. A summary of intervention characteristics is presented in Table 2. The duration of exercise interventions ranged from 2 to 12 months. One study did not report duration [55], one conducted the intervention for the duration of chemotherapy treatment and it continued for a further 6 weeks afterwards [60], and one commenced with chemotherapy and continued until symptoms of CIPN had subsided [53]. The type of exercise given to participants varied between studies, with some studies using a combination of resistance and aerobic exercises [51, 52, 54, 56, 57], combined strengthening, stretching, and balancing exercises [58], aerobic exercise via video recordings [59], or less strenuous physical training and sensorimotor exercises [60], Tibetan yoga [55], or nerve gliding exercises [53]. The frequency of interventions ranged from four sessions over the course of chemotherapy to three times daily, and session duration ranged from 5 to 90 min. Six studies provided some supervised sessions [51, 52, 54,55,56,57,58]. Nine of the studies had interventions during chemotherapy [53,54,55,56,57,58,59,60,61], and one began the intervention up to 1 week before chemotherapy [51, 52].
Quality of evidence
GRADE assessments showed that the quality of evidence for CIPN and HR-QoL was moderate. The meta-analysis data of both outcomes were judged to have, and downgraded for, serious imprecision due to low median sample size and a small number of included studies. The quality of evidence for fatigue was found to be very low. This was due to the high risk of bias found within one of the studies included in the fatigue meta-analysis, imprecision due to low sample size and a small number of included studies, and serious inconsistency of results. The results of the GRADE assessment can be found in Supplementary Material 4.
Risk of bias
The risk of bias was evaluated for all outcomes included in the review (CIPN, fatigue, and HR-QoL). A common concern was the lack of blinding of outcome assessors, which resulted in consistent judgement of some concerns due to possible deviations from the intended interventions. An additional common source of bias was due to missing data from participant drop-out and a failure to correct for, or identify, any potential bias. Three studies conducted appropriate intention-to-treat analyses [56, 57, 61]; however, only one detailed a method of imputation [56]. The risk of bias for the fatigue meta-analysis was judged to be high due to high risk of bias in a single included study [55]. This judgement was the result of a high participant attrition rate. A summary of the results of the risk of bias assessment can be found in Supplementary Material 5.
Effect on CIPN symptoms
The combined results of four RCTs [51,52,53, 58, 60] consisting of 20 effect estimates and 171 participants showed a reduction in CIPN symptoms following exercise compared with usual care (SMD − 0.71, 95% CI − 1.24 to − 0.17, p = 0.012; moderate-quality evidence; Fig. 2A). There was evidence of considerable heterogeneity (I2 = 76.9%, p < 0.001).
Forest plots of the results from multi-level random-effect meta-analyses on exercise intervention effects on A CIPN symptoms, B fatigue, and C HR-QoL. Data are presented as SMDs between exercise and usual care groups with corresponding 95% confidence intervals (95% CI). NPRS Numeric Pain Rating Scale, CIPNAT chemotherapy-induced peripheral neuropathy assessment tool, S-LANSS self-report version of leeds assessment for neuropathic symptoms and signs, CIPN20 The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire CIPN20, EORTC QLQ-30 The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-30, SF-36 36-Item Short Form Health Survey questionnaire, PFS Piper Fatigue Scale, MFSI-SF Multidimensional Fatigue Symptom Inventory-Short Form, MFI Multidimensional Fatigue Inventory, BFI Brief Fatigue Inventory
Effect on fatigue
The pooled results from seven RCTs [51, 52, 54,55,56,57, 59, 61] consisting of 8 effect estimates and 737 participants showed no difference in the levels of fatigue between exercise and usual care groups (SMD − 0.39, 95% CI − 0.95 to 0.18, p = 0.15; very low-quality evidence; Fig. 2B). There was evidence of considerable heterogeneity (I2 = 90.1%, p < 0.001).
Effect on HR-QoL
Based on the data from six RCTs [51, 52, 54, 56, 57, 59, 61] comprising 8 effect estimates and 609 participants, exercise interventions before and/or during taxane-containing chemotherapy regimens improved HR-QoL (SMD 0.42, 95% CI 0.07 to 0.76, p = 0.03; moderate-quality evidence; Fig. 2C). There was moderate heterogeneity of intervention effects (I2 = 49.6%, p = 0.06).
Adverse events
Five studies found no serious adverse events related to the exercise intervention [51, 52, 54,55,56, 60]. One study reported no difference in exercise adverse events between control and intervention [59]. One study reported no grade 3 or 4 toxicity in patients in relation to the intervention, but 2 types of adverse events (fatigue and myalgia or arthralgia) for whom it was difficult to determine their origin (cancer, chemotherapy, or intervention). Tendinitis and a calf snap may have been associated with the intervention [61]. Three studies did not report adverse events related to the intervention [53, 57, 58].
Sensitivity analysis
The use of a z distribution instead of a t distribution to compute the test statistics, change score SDs as the denominator in the calculation of SMDs instead of baseline SDs, and imputed change score SDs for the CIPN, fatigue, and HR-QoL meta-analyses, did not meaningfully influence the results. All sensitivity analyses can be found in Supplementary Material 6. The removal of each observation, in turn, had no significant impact on the effect estimate or level of heterogeneity in either the CIPN or fatigue meta-analysis. However, removing one effect estimate [54] changed the HR-QoL meta-analysis SMD so that it crossed the line of no effect. Removing Vincent et al. [61] from all meta-analyses, due to participants in that study receiving concomitant radiotherapy, did not impact the significance of any outcome. All results from the leave-one-out analysis are detailed in Supplementary Material 7.
Meta-regressions
Meta-regressions are presented in Supplementary Material 8. The covariates had a negligible influence on the level of heterogeneity. Meta-regressions were not undertaken for fatigue or HR-QoL effects because the meta-analyses included less than 10 effect estimates.
Discussion
This is the first study to synthesise data on the effects of exercise interventions before and/or during taxane-containing chemotherapy treatment on CIPN symptoms in women with breast cancer. This gives a unique insight into the potentially protective benefits of engaging in exercise during a taxane-containing chemotherapy regimen. Our findings show that performing exercise before and/or during taxane-containing regimens reduced symptoms of CIPN and improved HR-QoL. There was no evidence of an effect of exercise on fatigue. The evidence for CIPN and HR-QoL was judged to be of moderate quality, while the available evidence for the impact on fatigue was judged to be very low.
Several papers previously reported that exercise improves CIPN symptoms in patients with cancer [26]; however, previous evidence syntheses have not considered the potential therapeutic benefits of performing exercise before and/or during taxane-containing chemotherapy in women with breast cancer. Nevertheless, the finding that an exercise programme before and/or during taxane-containing chemotherapy regimens leads to higher levels of HR-QoL is consistent with the findings from a recent meta-analysis of RCTs that included a combination of treatment regimens, cancer types, and exercise intervention timings around chemotherapy (including after) [62].
The physiological mechanisms underpinning any preventive or attenuating effect of exercise on CIPN are currently unknown. However, the modelling of traumatic nerve injury in human and murine models has provided some mechanistic insight. For example, exercise has been shown to upregulate the expression of brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1 (IGF-1) [20], and the anti-inflammatory cytokines IL-10 and IL-1RA [63, 64], potentially indicating a pathway of alleviating the nerve damage and/or attenuating inflammation that has been implicated in the aetiology of CIPN and its symptoms [65]. Furthermore, taxanes have been shown to induce opening of mitochondrial permeability transition pores (MPTP) in axons, leading to the loss of membrane potential, reduced ATP, mitochondrial swelling, increased reactive oxygen species, and calcium release [66]. Exercise can increase the antioxidant capacity and electron transport chain efficiency of mitochondria, and preclinical studies have shown that acute exercise increases the ability of mitochondria to accumulate Ca2+ before opening MPTP [22]. The understanding of psychosocial mechanisms underpinning any effect of exercise on CIPN is perhaps limited to the reported associations between exercise and mental health (e.g. improved mood, anxiety, depression) [65]. In this respect, exercise may exert some of its influence on CIPN by modulating the known relationship between CIPN, fatigue, anxiety, and depression [67, 68]. Furthermore, because pre-treatment anxiety is associated with higher incidence of CIPN development [68], reducing pre-treatment anxiety via exercise performed before and/or during treatment may at least partially explain any effect observed.
Although this systematic review gives a unique insight into the impact of exercise before and/or during chemotherapy, it has some important limitations. There were some minor deviations from the pre-registered protocol, all of which have been documented and justified in Supplementary Material 1. Additionally, a limited number of studies were eligible to be included in this review, which demonstrates a problematic lack of research into exercise before and/or during taxane-containing chemotherapy regimens on CIPN, fatigue, and HR-QoL. The risk of bias also highlighted some concerns in both the CIPN and HR-QoL meta-analyses, partly due to a lack of blinding. This factor is challenging to overcome due to the context of the research. However, although blinding is impossible, investigations into any potential bias that this may cause would be beneficial. The quality of evidence for the fatigue meta-analysis was judged to be very low due to a high risk of bias and serious inconsistency. Furthermore, it must be noted that the significance of the output of the HR-QoL meta-analysis was reliant on the inclusion of a single effect estimate [54], suggesting the need for further research to increase the robustness of this outcome. Therefore, additional high-quality evidence is required to fully evaluate the impact of an exercise intervention before and/or during taxane-containing chemotherapy on fatigue and HR-QoL levels.
Furthermore, the diversity of interventions and outcome measurements makes the convergence of data from the included studies challenging and led to considerable between-study heterogeneity. The number of sessions ranged from 4 in total throughout chemotherapy to 21 times a week (3 sessions daily) and session duration ranged from 15 to 90 min. This limits the relevance of the effect point estimates, as there may be considerable variation in the effectiveness of interventions. A number of the included studies used interventions that were a combination of muscle strengthening and aerobic exercise [51, 52, 54, 56, 57, 61] or muscle strengthening and stretching exercises [58] while others used exercise interventions having a much lower intensity such as nerve gliding exercises [53] and yoga [55]. High levels of clinical heterogeneity could provide a partial explanation for the statistical heterogeneity observed in all three meta-analyses. High clinical heterogeneity can also lead to inaccurate conclusions and ultimately mislead decision-making [69]. Finally, the diversity of outcome measurements could limit the power of combined results. The small number of eligible studies rendered pooling only those studies that had the same outcome measures impossible. However, we chose a priori to incorporate potential heterogeneity into a random effects model under the assumption that the effects of exercise on different CIPN symptoms would be different, yet related, and would follow a normal distribution. The issue of varied and subjective CIPN measurements is one that appears in clinical practice. When interviewed, clinicians have previously expressed that one of the main barriers to CIPN assessment and management was ‘CIPN assessment practice patterns (e.g. use of subjective instead of objective CIPN assessment approaches)’ [70]. Therefore, increasing consistency in CIPN measurement will benefit research convergence as well as active clinical management. Future studies should focus on maximising evidence quality, by limiting the impact of missing data, working to reduce bias due to lack of participant blinding by blinding outcome assessors and data analysts, increasing the use of patient-centred measures, and striving towards a consistent and holistic CIPN measure.
A key strength of this evidence synthesis was the rigorous methodological approach, which included multiple sensitivity analyses of the main meta-analysis findings and a leave-one-out analysis, to individually assess the impact of each included observation. Additionally, heterogeneity was explored using meta-regressions were appropriate. The protocol and analysis plan were prospectively registered in the PROSPERO prospective register of systematic reviews (ref: CRD42021272036), and the search results, data, and statistical code are publicly available on OSF. Furthermore, we did not restrict the literature search to manuscripts only available in English, thus reducing the chance of missing any relevant studies written in other languages.
Conclusion
This review found reduced levels of CIPN symptoms and a higher HR-QoL in women with breast cancer who exercised before and/or during taxane-containing chemotherapy regimens, when compared to a usual care group. In contrast, there was no evidence of an effect of exercise on fatigue. Therefore, these results support the use of exercise, as an adjunct treatment before and/or during a taxane-containing treatment regimen for breast cancer, to reduce CIPN symptoms and improve HR-QoL.
Data availability
All data analysed during the meta-analyses and code used are available on the Open Science Framework (https://osf.io/bg896/?view_only=d70613ca97fb41689207a1a240b090df).
References
Pelster ADK, Coleman JD, Jawed-Wessel S, Irwin JA, Heerten-Rodriguez L, Fisher CM. Sexuality, breast cancer survivorship, and script theory. Sex Res Soc Pol. 2022. https://doi.org/10.1007/s13178-021-00672-w.
Broeckel JA, Jacobsen PB, Balducci L, Horton J, Lyman GH. Quality of life after adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2000;62(2):141–50. https://doi.org/10.1023/a:1006401914682.
Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33(1):E18-26. https://doi.org/10.1188/06.ONF.E18-E26.
Ghersi D, Wilcken N, Simes RJ. A systematic review of taxane-containing regimens for metastatic breast cancer. Br J Cancer. 2005;93(3):293–301. https://doi.org/10.1038/sj.bjc.6602680.
National Guideline Alliance (Great Britain). Early and locally advanced breast cancer: diagnosis and management. National Institute for Health and Care Excellence. 2018.
Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20(6):1451. https://doi.org/10.3390/ijms20061451.
Tofthagen C, McAllister RD, Visovsky C. Peripheral neuropathy caused by paclitaxel and docetaxel: an evaluation and comparison of symptoms. J Adv Pract Oncol. 2013;4(4):204–15.
Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav. 2017;7(1):e00558. https://doi.org/10.1002/brb3.558.
Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer. 2014;22(7):1999–2007. https://doi.org/10.1007/s00520-014-2242-z.
Bhatnagar B, Gilmore S, Goloubeva O, Pelser C, Medeiros M, Chumsri S, et al. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus. 2014;3:366. https://doi.org/10.1186/2193-1801-3-366.
Kirca K, Kutluturkan S. Symptoms experience and quality of life in the patients with breast cancer receiving the taxane class of drugs. Eur J Breast Health. 2018;14(3):148–55. https://doi.org/10.5152/ejbh.2018.3785.
Akcay D, Gozum S. Evaluation of the effect of education of chemotherapy side effects and home follow-up on the quality of life in patients with breast cancer given chemotherapy. Eur J Breast Health. 2021;8(4):191–9.
Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based profiles registry. J Clin Oncol. 2013;31(21):2699–707. https://doi.org/10.1200/JCO.2013.49.1514.
Beijers A, Mols F, Dercksen W, Driessen C, Vreugdenhil G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol. 2014;12(11):401–6. https://doi.org/10.12788/jcso.0086.
Hershman DL, Lacchetti C, Loprinzi CL. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2014;10(6):e421–4. https://doi.org/10.1200/JOP.2014.001776.
Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38(28):3325–48. https://doi.org/10.1200/JCO.20.01399.
Meng J, Zhang Q, Yang C, Xiao L, Xue Z, Zhu J. Duloxetine, a balanced serotonin-norepinephrine reuptake inhibitor, improves painful chemotherapy-induced peripheral neuropathy by inhibiting activation of p38 mapk and nf-kappab. Front Pharmacol. 2019;10:365. https://doi.org/10.3389/fphar.2019.00365.
Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–67. https://doi.org/10.1001/jama.2013.2813.
Chow R, Novosel M, So OW, Bellampalli S, Xiang J, Boldt G, et al. Duloxetine for prevention and treatment of chemotherapy-induced peripheral neuropathy (cipn): systematic review and meta-analysis. BMJ Support Palliat Care. 2022. https://doi.org/10.1136/spcare-2022-003815.
Park JS, Hoke A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS One. 2014;9(3):e90245. https://doi.org/10.1371/journal.pone.0090245.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15. https://doi.org/10.1038/nri3041.
Ascensao A, Lumini-Oliveira J, Machado NG, Ferreira RM, Goncalves IO, Moreira AC, et al. Acute exercise protects against calcium-induced cardiac mitochondrial permeability transition pore opening in doxorubicin-treated rats. Clin Sci (Lond). 2011;120(1):37–49. https://doi.org/10.1042/CS20100254.
Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett. 2015;596:90–107. https://doi.org/10.1016/j.neulet.2014.10.014.
Kidd JF, Pilkington MF, Schell MJ, Fogarty KE, Skepper JN, Taylor CW, et al. Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. J Biol Chem. 2002;277(8):6504–10. https://doi.org/10.1074/jbc.M106802200.
Duregon F, Vendramin B, Bullo V, Gobbo S, Cugusi L, Di Blasio A, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90–100. https://doi.org/10.1016/j.critrevonc.2017.11.002.
Lin WL, Wang RH, Chou FH, Feng IJ, Fang CJ, Wang HH. The effects of exercise on chemotherapy-induced peripheral neuropathy symptoms in cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2021;29(9):5303–11. https://doi.org/10.1007/s00520-021-06082-3.
Guo S, Han W, Wang P, Wang X, Fang X. Effects of exercise on chemotherapy-induced peripheral neuropathy in cancer patients: a systematic review and meta-analysis. J Cancer Surviv. 2022. https://doi.org/10.1007/s11764-022-01182-3.
Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001. https://doi.org/10.1002/14651858.CD005001.pub3.
Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6:135–47. https://doi.org/10.2147/CMAR.S44261.
Camara RJA, Schwentner L, Friedl TWP, Deniz M, Fink V, Lato K, et al. Quality of life during and after adjuvant anthracycline-taxane-based chemotherapy with or without gemcitabine in high-risk early breast cancer: results of the success a trial. Breast Cancer Res Treat. 2019;175(3):627–35. https://doi.org/10.1007/s10549-019-05171-6.
Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81(6):772–81. https://doi.org/10.1002/ana.24951.
Kerckhove N, Collin A, Conde S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol. 2017;8:86. https://doi.org/10.3389/fphar.2017.00086.
Loibl S, Skacel T, Nekljudova V, Luck HJ, Schwenkglenks M, Brodowicz T, et al. Evaluating the impact of relative total dose intensity (rtdi) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC Cancer. 2011;11:131. https://doi.org/10.1186/1471-2407-11-131.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74(9):790–9. https://doi.org/10.1016/j.rec.2021.07.010.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
National Cancer Institute. Nerve problems (peripheral neuropathy) and cancer treatment [Internet]. Maryland: NCI; c2020 [reviewed Jan 2020; cited 12 Jun 2023. Available from: https://www.cancer.gov/about-cancer/treatment/side-effects/nerve-problems.
Desforges AD, Hebert CM, Spence AL, Reid B, Dhaibar HA, Cruz-Topete D, et al. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: an update. Biomed Pharmacother. 2022;147:112671. https://doi.org/10.1016/j.biopha.2022.112671.
Maihofner C, Diel I, Tesch H, Quandel T, Baron R. Chemotherapy-induced peripheral neuropathy (cipn): current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer. 2021;29(8):4223–38. https://doi.org/10.1007/s00520-021-06042-x.
Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20(3):583–9. https://doi.org/10.1007/s00520-011-1127-7.
Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35(23):2604–12. https://doi.org/10.1200/JCO.2016.71.3552.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Chapter 8: assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from https://www.training.cochrane.org/handbook.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organizational research methods. 2008;11(2):364–86. https://doi.org/10.1177/1094428106291059.
Cohen J. Statistical power analysis for the behavioral sciences. Academic press. 2013.
Rover C, Knapp G, Friede T. Hartung-knapp-sidik-jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15:99. https://doi.org/10.1186/s12874-015-0091-1.
Van den Noortgate W, Lopez-Lopez JA, Marin-Martinez F, Sanchez-Meca J. Three-level meta-analysis of dependent effect sizes. Behav Res Methods. 2013;45(2):576–94. https://doi.org/10.3758/s13428-012-0261-6.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Chapter 10: analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022. Available from https://www.training.cochrane.org/handbook.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Chapter 16 special topics in statistics. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022. Available from https://www.training.cochrane.org/handbook.
Orange ST, Hicks KM, Saxton JM. Effectiveness of diet and physical activity interventions amongst adults attending colorectal and breast cancer screening: a systematic review and meta-analysis. Cancer Causes Control. 2021;32(1):13–26. https://doi.org/10.1007/s10552-020-01362-5.
Bland KA, Kirkham AA, Bovard J, Shenkier T, Zucker D, McKenzie DC, et al. Effect of exercise on taxane chemotherapy-induced peripheral neuropathy in women with breast cancer: a randomized controlled trial. Clin Breast Cancer. 2019;19(6):411–22. https://doi.org/10.1016/j.clbc.2019.05.013.
Kirkham AA, Bland KA, Zucker DS, Bovard J, Shenkier T, McKenzie DC, et al. Chemotherapy-periodized’ exercise to accommodate for cyclical variation in fatigue. Med Sci Sports Exerc. 2020;52(2):278–86. https://doi.org/10.1249/MSS.0000000000002151.
Andersen Hammond E, Pitz M, Steinfeld K, Lambert P, Shay B. An exploratory randomized trial of physical therapy for the treatment of chemotherapy-induced peripheral neuropathy. Neurorehabil Neural Repair. 2020;34(3):235–46. https://doi.org/10.1177/1545968319899918.
Carayol M, Ninot G, Senesse P, Bleuse JP, Gourgou S, Sancho-Garnier H, et al. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: the ‘apad1’ randomized controlled trial. BMC Cancer. 2019;19(1):737. https://doi.org/10.1186/s12885-019-5896-6.
Chaoul A, Milbury K, Spelman A, Basen-Engquist K, Hall MH, Wei Q, et al. Randomized trial of tibetan yoga in patients with breast cancer undergoing chemotherapy. Cancer. 2018;124(1):36–45. https://doi.org/10.1002/cncr.30938.
Cornette T, Vincent F, Mandigout S, Antonini MT, Leobon S, Labrunie A, et al. Effects of home-based exercise training on vo2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (sapa): a randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(2):223–32.
Jacot W, Arnaud A, Jarlier M, Lefeuvre-Plesse C, Dalivoust P, Senesse P, et al. Brief hospital supervision of exercise and diet during adjuvant breast cancer therapy is not enough to relieve fatigue: a multicenter randomized controlled trial. Nutrients. 2020;12(10):3081. https://doi.org/10.3390/nu12103081.
Simsek NY, Demir A. Cold application and exercise on development of peripheral neuropathy during taxane chemotherapy in breast cancer patients: a randomized controlled trial. Asia Pac J Oncol Nurs. 2021;8(3):255–66. https://doi.org/10.4103/apjon.apjon-2075.
Sturgeon KM, Smith AM, Federici EH, Kodali N, Kessler R, Wyluda E, et al. Feasibility of a tailored home-based exercise intervention during neoadjuvant chemotherapy in breast cancer patients. BMC Sports Sci Med Rehabil. 2022;14(1):31. https://doi.org/10.1186/s13102-022-00420-6.
Vollmers PL, Mundhenke C, Maass N, Bauerschlag D, Kratzenstein S, Rocken C, et al. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J Cancer Res Clin Oncol. 2018;144(9):1785–92. https://doi.org/10.1007/s00432-018-2686-5.
Vincent F, Deluche E, Bonis J, Leobon S, Antonini MT, Laval C, et al. Home-based physical activity in patients with breast cancer: during and/or after chemotherapy? Impact on cardiorespiratory fitness. A 3-arm randomized controlled trial (apac). Integr Cancer Ther. 2020;19:1534735420969818. https://doi.org/10.1177/1534735420969818.
Lopez-Garzon M, Cantarero-Villanueva I, Postigo-Martin P, Gonzalez-Santos A, Lozano-Lozano M, Galiano-Castillo N. Can physical exercise prevent chemotherapy-induced peripheral neuropathy in patients with cancer? A systematic review and meta-analysis. Arch Phys Med Rehabil. 2022;103(11):2197–208. https://doi.org/10.1016/j.apmr.2022.02.008.
Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33.
Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. Il-6 enhances plasma il-1ra, il-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–7. https://doi.org/10.1152/ajpendo.00074.2003.
Chung KH, Park SB, Streckmann F, Wiskemann J, Mohile N, Kleckner AS, et al. Mechanisms, mediators, and moderators of the effects of exercise on chemotherapy-induced peripheral neuropathy. Cancers (Basel). 2022;14(5):1224. https://doi.org/10.3390/cancers14051224.
Canta A, Pozzi E, Carozzi VA. Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (cipn). Toxics. 2015;3(2):198–223. https://doi.org/10.3390/toxics3020198.
Kleckner IR, Jusko TA, Culakova E, Chung K, Kleckner AS, Asare M, et al. Longitudinal study of inflammatory, behavioral, clinical, and psychosocial risk factors for chemotherapy-induced peripheral neuropathy. Breast Cancer Res Treat. 2021;189(2):521–32. https://doi.org/10.1007/s10549-021-06304-6.
Lee KM, Jung D, Hwang H, Son KL, Kim TY, Im SA, et al. Pre-treatment anxiety is associated with persistent chemotherapy-induced peripheral neuropathy in women treated with neoadjuvant chemotherapy for breast cancer. J Psychosom Res. 2018;108:14–9. https://doi.org/10.1016/j.jpsychores.2018.02.012.
Gagnier JJ, Moher D, Boon H, Beyene J, Bombardier C. Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Methodol. 2012;12:111. https://doi.org/10.1186/1471-2288-12-111.
Knoerl R, Wallar J, Fox E, Hong F, Salehi E, McCleary N, et al. Exploring clinicians’ perspectives of barriers to chemotherapy-induced peripheral neuropathy assessment and management in oncology practice: a qualitative analysis of semi-structured interviews. Cancer Nurs. 2022. https://doi.org/10.1097/NCC.0000000000001082.
Acknowledgements
This work was supported by Northumbria University.
Funding
This paper presents independent research funded by Northumbria University. Author RB-S has received research support from Northumbria University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Database searching, data extraction, and quality assessment were performed by RB-S and JT. Data analysis was performed by RB-S and STO. The first draught of the manuscript was written by RB-S, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to participate
Informed consent was not applicable for this review.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brownson-Smith, R., Orange, S.T., Cresti, N. et al. Effect of exercise before and/or during taxane-containing chemotherapy treatment on chemotherapy-induced peripheral neuropathy symptoms in women with breast cancer: systematic review and meta-analysis. J Cancer Surviv (2023). https://doi.org/10.1007/s11764-023-01450-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-023-01450-w