Abstract
Purpose
Breast lymphoedema is a possible side effect of breast conserving surgery, but it is poorly understood. This is due, in part, to difficulty assessing the breast. This systematic review described outcome measures that quantify breast lymphoedema signs and symptoms and evaluated the measurement properties for these outcome measures.
Method
Seven databases were searched using terms in four categories: breast cancer, lymphoedema and oedema, clinician reported (ClinROM) and patient reported outcome measures (PROM) and psychometric and measurement properties. Two reviewers independently reviewed studies and completed quality assessments. The Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) methodology was used for studies including measurement property evidence.
Results
Fifty-six papers were included with thirteen questionnaires, eight patient-reported rating scales, seven physical measures, seven clinician-rating scales and four imaging techniques used to quantify breast lymphoedema. Based on COSMIN methodology, one ClinROM had sufficient reliability, ultrasound measuring dermal thickness. Tissue dielectric constant (TDC) measuring local tissue water had promising reliability. Four questionnaires had sufficient content validity (BLYSS, BLSQ, BrEQ and LYMQOL-Breast).
Conclusions
Ultrasound is recommended to reliably assess breast lymphoedema signs. No PROM can be recommended with confidence, but BLYSS, BLSQ, BrEQ and LYMQOL-Breast are promising. Further research is recommended to improve evidence of measurement properties for outcome measures.
Implications for Cancer Survivors
There are many approaches to assess breast lymphoedema, but currently, only ultrasound can be recommended for use, with others, such as TDC and questionnaires, showing promise. Further research is required for all approaches to improve evidence of measurement properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast conserving surgery with adjuvant radiotherapy is a common treatment regimen for women with early breast cancer as it leads to better quality of life [1] and improved survival to that of women undergoing mastectomy [2, 3]. Unfortunately, breast lymphoedema can be a painful and distressing complication of the breast conserving treatment regime [4, 5]. Breast lymphoedema is not well understood and poorly addressed by health professionals [6]. The reported incidence of breast lymphoedema varies considerably across studies, ranging from 0 to 90% due to variances in the definition and tools selected to diagnose and quantify breast lymphoedema [7, 8].
Assessments of lymphoedema in the limbs have been validated [9,10,11,12,13]; however, it is unknown if those tools can be used in the assessment of breast lymphoedema. Measurement of lymphoedema in the breast differs to that in the arm as the breast is the direct recipient of the surgical and radiotherapy treatment. These treatments change the volume and tissue architecture of the affected breast, reducing the usefulness of measuring the breast pre-operatively or measuring the contralateral breast as a direct comparator. Changes to the breast caused by surgery and radiotherapy may also make it more difficult to distinguish between treatment impacts and those changes caused by presence of breast lymphoedema. Furthermore, self-reported questionnaires for lymphoedema have tended to focus on and be tested with people with limb lymphoedema rather than on populations with breast or midline lymphoedema [11, 13].
This systematic review describes what outcome measures are available to quantify breast lymphoedema signs and symptoms following breast conserving surgery and evaluates the evidence underpinning the measurement properties for these assessment tools or approaches, where available.
Methods
The systematic review was registered with the International Prospective Register of Systematic Reviews on 05 July 2020 (PROSPERO registration no: CRD42020183851).
The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14] and Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) guideline for systematic reviews [15,16,17].
Database search
Five electronic databases were searched including Medline, Embase, CINAHL, Web of Science and Scopus as well as Trove and ProQuest Dissertations & Theses Global for theses that explored breast lymphoedema measurement. Searches were conducted with support from a librarian at the University of Sydney. Search terms were grouped into four categories relating to (i) breast cancer; (ii) lymphoedema and oedema; (iii) clinician-reported (ClinROM) and patient-reported outcome measures (PROM); and (iv) psychometric and measurement properties. The full Medline search strategy is described in Online Resource 1. The initial search was conducted on 19th April 2020 and repeated on 19th August 2021 and 14th February 2022 to check for recently published articles. There was no restriction on date of publications, but only articles published in English were included.
Selection criteria
Studies were included in which an assessment was used to quantify breast lymphoedema and related symptoms (e.g. peau d'orange, induration, hardness, heaviness, discomfort, skin redness) in adult women following breast conserving surgery (lumpectomy/wide local excision) for breast cancer. Women may have been treated with chemotherapy, radiotherapy and/or immunotherapy. Theses were included when publicly available online or provided by authors following request. Studies with men, women under 18 years old, women treated with mastectomy and/or reconstruction and assessment for lymphoedema in areas of the body other than the breast were excluded. Studies only using toxicity or cosmesis rating scales (e.g. CTCAE, LENT SOMA, National Cancer, Institute Canada-Common Toxicity Criteria 2, Harvard Breast Cosmesis Scale, Outcome by American Society for Radiation Oncology (ASTRO) Consensus Panel (CP) group and acute and late RTOG scales) were also excluded.
Study selection
Duplicates were removed using electronic and manual review in EndNOTE (version X9) with additional duplicates identified when titles were imported to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia (available at www.covidence.org). Titles and abstracts, followed by full text papers, were independently screened by two reviewers (NF, SK, CL). Reference lists of included full text papers were examined to identify additional appropriate studies. When disagreements on study eligibility occurred, consensus was reached through discussion as a team.
Data extraction and analysis
Two reviewers independently extracted data (SK and NF or CL and NF) using Covidence data extraction template (version 1). Information extracted included study design, participant demographics, treatment history and the stage at which the assessments took place in the participants’ cancer treatment timeline (e.g. time since diagnosis, surgery and/or radiotherapy). The purpose of the assessment (e.g. assessing treatment side effects, quality of life or measuring outcomes from an intervention to treat breast lymphoedema) and details pertaining to the measurement properties of the tools were also extracted where available. If there were missing data or data from participants following breast conserving surgery or breast lymphoedema were not presented separately, authors were contacted requesting this data.
Quality assessment
Several tools were used to assess the quality and risk of bias of included papers due to the variety of study designs included in this review. All assessments were completed by two reviewers (NF, SK, CL) independently and all disagreements were resolved through discussion until agreement was made. Included papers that had been authored by SK were assessed by other team members (NF and CL) to prevent potential bias in quality assessment.
The Cochrane tool for assessing risk of bias (RoB) in randomised trials, version 2 (RoB 2) [18], was used for the randomised controlled trials, and the quality assessment for cohort or non-randomised experimental studies was completed using the National Heart, Lung and Blood institute (NHLBI) Quality Assessment Tool for before-after (pre-post) studies with no control group (URL: www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed 26 October 2020).
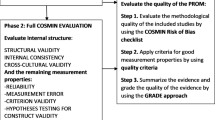
Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) Risk of Bias checklist adapted for clinical measures (ClinROMs) [15] was completed for studies including measurement properties for clinician rating scales, measurement device or imaging tool. The COSMIN ROB checklist for patient reported outcome measures (PROMs) [16] was used for studies including measurement properties for PROMs. The studies were assessed separately against each standard using the four-point scale (very good, adequate, doubtful or inadequate), and then quality was rated using the “worst-score-counts method” [17]. The quality of PROM development was evaluated first, followed by the quality of content validity studies, and these results were combined to rate the content validity overall based on relevance, comprehensiveness and comprehensibility for breast lymphoedema measurement in women following conservative breast cancer treatment. Next, the other eight measurement properties were evaluated. Finally, the overall quality of evidence for each tool was graded using the modified GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach incorporating the assessment of risk of bias, inconsistency, imprecision and indirectness to grade the quality of evidence as high, moderate, low or very low quality [17].
Recommendations for the use of tools or approaches were categorised, based on the evidence, as (A) recommended (PROM, evidence of sufficient content validity and internal consistency; ClinROM, evidence of sufficient face validity and reliability), (B) promising (additional validation studies required, not categorised as A or C) or (C) insufficient (high quality evidence of insufficient measurement property) [17].
Data synthesis
A narrative synthesis of the findings from the included studies was performed for the breast lymphoedema measurement tools or approaches and available measurement properties. Meta-analysis was not conducted as the review was of the assessment tools, not treatment outcomes or efficacy of treatment.
Results
The search of databases identified 7805 papers and 169 theses titles, with 2306 duplicates removed. Following title and abstract review, 156 papers progressed to full paper review. Fifty-four papers and two theses met the inclusion criteria for this review (Fig. 1) following review of the full papers. Thirty-two studies measured breast lymphoedema signs and symptoms as a side effect of cancer treatments including breast conserving surgery and radiotherapy [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. Six studies measured the outcome of specific breast lymphoedema interventions [51,52,53,54,55,56]. Seventeen studies reported on the measurement properties of the tools used and were further analysed with the COSMIN framework [31, 38, 57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram [14]
Fourteen studies used a combination of ClinROM and PROM, including all but one [52] of the breast lymphoedema interventions studies. ClinROMs alone were used in 23 studies, and PROMs alone in 20 studies. Most studies (62.5%) used at least two different tools, with one study using seven [59].
Characteristics of clinician reported outcome measures
Signs of breast lymphoedema were quantified using multiple tools and approaches (Table 1). Breast tissue dermal thickness was measured using ultrasound (n = 12) [21,22,23,24, 35, 36, 54, 57,58,59,60, 72] and mammography (n = 3) [26,27,28]; local tissue water was measured using tissue dielectric constant (TDC) (n = 8) [19, 20, 51,52,53, 59, 61, 73]; breast volume was measured using three-dimensional surface imagery (3D-SI: n = 4) [37, 40, 56, 63], magnetic resonance imaging (MRI: n = 1) [41] and anthropomorphic techniques (n = 1) [36]; extracellular fluid volume was measured using bioimpedance spectroscopy (BIS: n = 3) [54, 62, 74]; tissue resistance was measured using tonometry (n = 3) [55, 59, 62], the pitting test (n = 2) [59, 61] and indentation force (n = 1) [52]; and dermal backflow/compensatory drainage pathways was visualised with indocyanine green imaging (ICG: n = 1) [73]. Additionally, clinician rating scales (n = 8) [29, 31, 33,34,35, 37, 47, 72] were used to identify presence of changes to the appearance, size or texture of the breast tissue. Rating scales were also used by clinicians to identify or grade indicators of breast lymphoedema seen using ultrasound or mammography, including signs of parenchymal or cutaneous oedema, trabecular thickening and skin elasticity (n = 8) [24,25,26,27,28,29, 35, 36].
Measurement locations for the different tools and techniques varied and were either taken of the entire breast, quadrants or one or two selected locations on the breast. The entire breast was assessed for dermal backflow (ICG), volume (3D-SI, anthropomorphic, and MRI) and clinical rating scales. Ultrasound (dermal thickness), BIS, tissue resistance and TDC measures were performed both in breast quadrants [35, 36, 53, 57,58,59,60, 62, 73], two breast sites [21,22,23,24] or a single measurement site [52, 54, 55, 61, 72, 74]. TDC breast quadrant measures were also combined and reported as averages [19, 20, 51, 53, 59, 61] with the unaffected breast that was assessed to determine ratios. BIS measures were also reported as a ratio for the affected breast compared to the unaffected breast [54, 74].
COSMIN summary: ClinROMs
The COSMIN Risk of Bias tool adapted for clinical measures (ClinROMs) [15] was completed for five clinical assessment tools [31, 57, 59,60,61,62,63], from eight studies that evaluated measurement properties. Evaluation of face validity, reliability and measurement error were conducted for all tools (Table 2). Criterion validity was not evaluated as there is no gold standard for measurement of breast lymphoedema. Measurement properties for dermal thickness measured with mammography as well as imaging signs and breast volume measured using anthropomorphic or MRI techniques were not reported in the studies meeting the inclusion criteria for this study. Only a single study presented measurement properties for clinician rating scales [31].
Face validity was evaluated as sufficient for dermal thickness measurement using ultrasound [57, 59, 60], local tissue water measured with TDC [59, 61], breast volume measurement using 3D-SI [63], extracellular fluid measured with BIS [62], tissue resistance measured with pitting [61] and clinician rating scales of breast lymphoedema signs [31]. Tonometry face validity was evaluated as being indeterminate [59, 62] and having insufficient structural validity as this tool could not detect a difference between affected and unaffected breasts or lymphoedematous and non-lymphoedematous breasts [59]. Structural validity was not described for any other ClinROMs.
Reliability was rated as sufficient for measurement of dermal thickness for both the image capture [60] and image measurement [57, 59] using ultrasound, with a GRADE rating of moderate quality of evidence, due, in part, to low combined sample size (< 100) of studies that investigated it. Reliability was also evaluated as sufficient for TDC measuring percentage water content (PWC) ratio (affected:unaffected breasts) [59, 61]; however, it received a GRADE rating of low quality evidence due to imprecision (combined sample size for two studies < 50). Reliability of a clinician rating scale was indeterminate from a single study with GRADE rating downgraded to low quality due to risk of bias [31]. Pitting test reliability was rated as insufficient based on results from a single study with a GRADE of low quality due to small sample size (< 50) [59]. Reliability results were not available for tonometry, BIS, ICG or breast volume measurement.
Measurement error for all assessment tools was graded as indeterminate as minimally important change (MIC) has not been defined for any breast lymphoedema tools. Both dermal thickness assessed by ultrasound [57, 59, 60] and TDC [59, 61] had values for standard error of measurement and limits of agreement to allow some interpretation of results, with quality of evidence for measurement error graded as moderate for dermal thickness assessed by ultrasound and low for TDC, both of which were downgraded for the same reasons described for reliability respectively. Coefficient of variation was reported for tonometry [62], BIS [62] and breast volume measured by 3D-SI [63] with the quality of evidence for these tools graded as very low. There was no measurement error information for pitting test [59, 61] or clinician rating scales [31].
Based on the information provided, dermal thickness measurement assessed by ultrasound is recommended (Category A) for the assessment of breast lymphoedema as it has both have sufficient face validity and evidence for sufficient reliability with moderate quality of evidence. The other assessment tools, including TDC, BIS, tonometry, 3D-SI, clinician rating scales and the pitting test, are categorised as promising (Category B) as they do not have sufficient evidence for reliability, and the studies are of low or very low quality. No tools were categorised as insufficient (Category C).
Characteristics of PROMs
Patient-reported breast lymphoedema symptoms severity and/or intensity were quantified in 14 studies with seven using questionnaires (Breast Lymphoedema Symptom Severity (BLYSS), Breast Edema Questionnaire (BrEQ), Disabilities of the Arm, Shoulder and Hand (Modified DASH), BSQ-Breast Symptom Questionnaire (BSQ), Lymphedema Symptom Intensity and Distress Survey-Trunk (LSIDS-T), Breast Cosmesis Questionnaire (BCQ), Breast Lymphoedema Symptom Questionnaire (BLSQ) and Breast Symptom Scale (BSS) [32, 54, 55, 58, 59, 64, 72]), six using visual analogue scales (VAS) [31, 48, 51, 53, 55, 56] and one using yes/no response options [73] (Table 1). Further, five questionnaires, European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life Questionnaire (EORTC-QLQ BR23), Breast Cancer Treatment Outcome Scale-22, -13 and -12 (BCTOS-22, BCTOS-13, BCTOS-12) and Lymphoedema Quality of Life tool-Breast (LYMQOL-Breast), were used to quantify quality of life (QOL) as related to breast lymphoedema or associated symptoms in 24 studies. The EORTC QLQ BR23 was partly completed in three studies [41, 54, 70], using only the breast symptoms subscale with or without the arm symptom subscale, which measured symptoms rather than QOL, per se. The BCOTS-22, BCOTS-13 and BCOTS-12 had conflicting reporting of the construct it measured, with some studies reporting the subscales separately and used the subscales as a measure of cosmesis, pain and/or function [30, 38,39,40,41,42, 45, 46, 49, 50, 66, 67, 69,70,71], while others combined the subscales to measure QOL [47, 48, 65, 68].
COSMIN-PROM
COSMIN for PROM [16] was used to evaluate nine PROMs from eleven papers [38, 58, 59, 64,65,66,67,68,69,70,71] meeting the inclusion criteria for this systematic review as well as two additional original validation papers in mixed breast cancer populations [75, 76] (Table 3).
All included PROMs were evaluated as having adequate face validity; however, all PROM development studies lacked the detail required by the COSMIN methodology to score above a rating of doubtful quality. The BLYSS, BrEQ and EORTC-BR23 received a doubtful rating for quality of PROM design and the pilot study and a doubtful rating overall for PROM development. These three questionnaires consulted patients for concept elicitation using a qualitative approach but lacked detail on the interview and analysis process. Authors for the BSLQ and LYMQOL-Breast involved patients using quantitative methods for concept elicitation and to confirm comprehensibility and comprehensiveness but only performed this with a small sample of women (n = 20) resulting in an inadequate quality rating for PROM development.
The BCTOS-22/12/13 and LSIDS-T were all rated as inadequate for PROM design as they did not involve patients, either relying on literature review and experience of the authors (BCTOS), or only involving professionals in PROM design and pilot testing (LSIDS-T). A single exception was the pilot study testing the BCTOS-13 [70]. This study involved patients to rate comprehensibility and relevance resulting in a doubtful rating for quality for this pilot study.
The content validity studies for the nine PROMs similarly only achieved a maximum rating of doubtful quality. The BLYSS, LYMQOL-Breast, BLSQ, EORTC-BR23 and BrEQ were all rated as doubtful quality for relevance, comprehensiveness and comprehensibility due to lack of detail on the conduct and analysis of patient or professional interviews (BLYSS/EORTC-BR23/BrEQ) or only surveys being used (BLSQ, LYMQOL-Breast). The original studies for BCTOS were rated as inadequate quality for content validity. However, the German [69] and Brazilian-Portuguese [68] translations of BCTOS-22 did ask patients regarding comprehensibility but was rated as doubtful quality, due to limited information on analysis of this process. LSIDS-T content validity was also rated as inadequate due to lack of patient involvement in the content validity study.
Overall, BLYSS had sufficient quality of evidence with moderate grade evidence for content validity. The BrEQ, BLSQ and LYMQOL-Breast were also rated as sufficient with moderate GRADE evidence, with downgrading due to either lack of input from professionals (BrEQ) or use of quantitative methods in only a small sample for patient feedback (BLSQ/LYMQOL). The EORTC-BR23 had sufficient quality of evidence with low GRADE evidence due to indirectness of the sample used. LSIDS-T and BCTOS were both rated as indeterminate with very low GRADE evidence for content validity.
Six of the nine measurement properties were reported for the included PROMs (Table 3). Construct validity was evaluated for all questionnaires with a sufficient rating for six questionnaires (BrEQ, BCTOS-12, BLSQ, LYMQOL-Breast, EORTC QLQ BR23 and LSIDS-T). Internal consistency was evaluated for six questionnaires (BrEQ, BCTOS-22, BCTOS-12, BCTOS-13, LYMQOL-Breast, EORTC-BR23, LSIDS-T) with two measures receiving a sufficient rating (BCTOS-22, BCTOS-12) with high GRADE evidence. Reliability was evaluated for seven questionnaires (BrEQ, BLYSS, BCTOS-22 [Brazilian-Portuguese], BCTOS-13, BLSQ, LYMQOL-Breast, EORTC-BR23), with five achieving a sufficient rating (BrEQ, BLYSS, BCTOS-22, BCTOS-13, BLSQ), but the GRADE was low or very low for all. Structural validity was evaluated for four questionnaires (BCTOS-22, BCTOS-13, BCTOS-12, LSIDS-T), but no measure achieved a sufficient rating for this measurement property. The BCTOS-22 had an insufficient rating with high GRADE evidence. Responsiveness was only available for two questionnaires (BCTOS-22 and EORTC-BR23 [Spanish and Dutch versions]), with the EORTC-BR23 [Spanish and Dutch versions] achieving a sufficient rating with low GRADE evidence, and the BCTOS-22 rated as insufficient with very low GRADE evidence. Measurement error was presented for just one questionnaire (LYMQOL-Breast) and was rated as indeterminate with low GRADE evidence. Cross-cultural validity, criterion validity and measurement invariance were not presented for any questionnaires. There was no gold standard to assess criterion validity for PROMs.
Quality assessment
Seven randomised controlled trials were included and assessed using the RoB 2 tool [18] (Table 1) [39, 40, 46, 51, 53, 54, 56]. Three RCTs had low overall risk of bias [46, 53, 54], and four were rated as having some concerns [39, 40, 51, 56]. Quality assessment for the 49 non-randomised studies [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38, 41,42,43,44,45, 47,48,49,50, 52, 55, 57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] was completed using the NHLBI quality assessment tool (URL: www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [accessed 26 October 2020]). Seven studies were rated as good quality [19, 45, 47, 49, 58, 60, 74], 25 rated as fair [20,21,22,23,24, 26, 27, 30,31,32, 35, 36, 42, 57, 59, 61, 63,64,65,66,67, 70,71,72,73] and 17 as poor [25, 28, 29, 33, 34, 37, 38, 41, 43, 44, 48, 50, 52, 55, 62, 68, 69] (Table 1).
Discussion
The signs and symptoms of breast lymphoedema were quantified using a variety of approaches, including 13 patient-reported questionnaires, eight patient-reported rating scales, seven types of physical measures, seven clinician rating scales and four imaging techniques. Dermal thickness measured with ultrasound is recommended for assessment of breast lymphoedema, but further studies are required to establish the MCID and responsiveness (the validity of a change score). A breast lymphoedema PROM, however, cannot be recommended at this time as the reported details for development and measurement properties were lacking for all questionnaires. Nevertheless, the symptom-based PROMs, BLYSS, BLSQ and BrEQ (Dutch) and the QOL PROM LYMQOL-Breast are promising, with sufficient content validity. However, all tools require additional appropriately powered studies with women with, or at risk of breast lymphoedema to improve the measurement property evidence.
To fully assess the impact of breast lymphoedema, more than one assessment tool is suggested [7, 54, 77]. Breast lymphoedema is complex, with no agreed upon definition of the condition and with the presence of oedema in the breast influenced by treatment factors including surgery, radiotherapy and chemotherapy [78]. Measurement of signs and symptoms of breast lymphoedema, including both clinician- and patient-reported outcomes, would provide a comprehensive assessment of the underlying changes occurring. Forty-six percent of the included studies in this systematic review assessed more than one measurement outcome to quantify breast lymphoedema with 14 reporting both patient-reported and clinician-reported outcomes [31, 36, 40, 41, 47, 51, 53,54,55,56, 58, 59, 72, 73]. Inclusion of both ClinROMs and PROMs can also highlight the discord between patient and clinician reported outcomes, such as has been found in arm lymphoedema [12, 79], For example, measurements of dermal thickness provided information on the secondary tissue changes that can occur within the oedematous breast, but this does not necessarily relate to symptoms experienced by women [60]. Furthermore, due to the lack of a gold standard to assess the tools, we are unable to determine which tool is the best. Therefore, use of multiple tools, including those tools with the best available measurement property evidence, are recommended.
The practicality and expense of tools to quantify breast lymphoedema is a consideration for clinical usefulness. Questionnaires are the least expensive option, but responsiveness has only been established in EORTC-BR23 in non-English speaking samples. Ultrasound is readily available in hospitals and imaging centres but may be less accessible in private clinics where lymphoedema therapists often treat patients with lymphoedema. Comparably, TDC is a small, portable tool that could prove useful in clinical settings, but cost may still be prohibitive for small clinics at approximately $6000 AUD for a unit. Unfortunately, two reliable approaches that are widely used for limb lymphoedema, volume measurement and BIS [12], do not currently have sufficient evidence for breast lymphoedema assessment.
This review highlighted the need for standardised assessment protocols for the ClinROMs as there was heterogeneity across many of the studies on the measurement locations on the breast, with some studies reporting individual quadrant results while others only reporting overall means/ratios. For example, findings for dermal thickness measured with ultrasound and TDC may have been influenced by the location at which the measurement was taken. In healthy breasts, dermal thickness is greater in the inferior and medial breast quadrants [57, 59]; similarly, TDC varied across location in healthy breasts as well as unaffected breasts [59, 73]. Other factors such as age and menopausal status [80] and scar tissue [81] may also impact on breast signs but have yet to be investigated in the context of women with breast lymphoedema. Inclusion of these data may become important in the future in interpreting the findings.
This review identified significant gaps for the measurement properties of breast lymphoedema tools. The COSMIN framework for determining ratings for measurement properties is very comprehensive and relies on studies thoroughly reporting the study design to avoid poor ratings. Nevertheless, overall, there was a lack of high-quality evidence of measurement properties for breast lymphoedema tools. Dermal thickness measured with ultrasound had the most evidence but still lacked evidence of measurement error due to no established MCID for these or any breast lymphoedema tools. Four questionnaires (BrEQ, BLYSS, BLSQ, LYMQOL-Breast) were promising but require further investigation and larger sample sizes to improve overall quality of evidence for their measurement properties and overall quality of evidence. It is only after those investigations can recommendations to be made about their usefulness in assessment of breast lymphoedema.
Conclusion
The findings from this systematic review reveal that ultrasound has the best measurement properties, including information on measurement error, but MIC has not yet been established. Of the PROMS, BLYSS, BrEQ and BLSQ for symptom severity and LYMQOL-Breast for measurement of QOL are promising tools to assess women following conservative breast cancer treatment. Well-designed and reported studies on measurement properties for all tools are required to improve quality of evidence in this emerging area of assessment. Based on the current level of evidence, a combination of objective and subjective measurements is recommended to quantify the full manifestation of breast lymphoedema signs and symptoms.
Systematic Review Registration
This systematic review was registered in PROSPERO (CRD42020183851).
References
Zehra S, Doyle F, Barry M, et al. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: a systematic review and meta-analysis. Breast Cancer. 2020;27(4):534–66. https://doi.org/10.1007/s12282-020-01076-1.
Christiansen P, Mele M, Bodilsen A, et al. Breast-conserving surgery or mastectomy?: Impact on survival. Ann Surg Open. 2022;3(4):e205.
Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149(3):267–74. https://doi.org/10.1001/jamasurg.2013.3049.
Todd M. Identification, assessment and management of breast oedema after treatment for cancer. Int J of Palliat Nurs. 2017;23(9):440–4. https://doi.org/10.12968/ijpn.2017.23.9.440.
Doersam JK, Dietrich MS, Adair MA, et al. A comparison of symptoms among patients with head and neck or truncal lymphedema and normal controls. Lymphat Res Biol. 2019;17(6):661–70. https://doi.org/10.1089/lrb.2019.0034.
Probst H, Rosbottom K, Crank H, et al. The patient experience of radiotherapy for breast cancer: a qualitative investigation as part of the SuPPORT 4 All study. Radiography. 2021;27(2):352–9. https://doi.org/10.1016/j.radi.2020.09.011.
Verbelen H, Gebruers N, Beyers T, et al. Breast edema in breast cancer patients following breast-conserving surgery and radiotherapy: a systematic review [Review]. Breast Cancer Res Treat. 2014;147(3):463–71. https://doi.org/10.1007/s10549-014-3110-8.
Abouelazayem M, Elkorety M, Monib S. Breast lymphedema after conservative breast surgery: an up-to-date systematic review. Clin Breast Cancer. 2021;21(3):156–61. https://doi.org/10.1016/j.clbc.2020.11.017.
Hidding JT, Viehoff PB, Beurskens CHG, et al. Measurement properties of instruments for measuring of lymphedema: systematic review. Phys Ther. 2016;96(12):1965–81. https://doi.org/10.2522/ptj.20150412.
Llanos C, Gan EY, Chen J, et al. Reliability and validity of physical tools and measurement methods to quantify hand swelling: a systematic review. Phys Ther. 2021;101(2):pzaa206. https://doi.org/10.1093/ptj/pzaa206.
Paramanandam VS, Lee M-J, Kilbreath SL, et al. Self-reported questionnaires for lymphoedema: a systematic review of measurement properties using COSMIN framework. Acta Oncol. 2021;60(3):379–91. https://doi.org/10.1080/0284186X.2020.1862422.
Czerniec SA, Ward LC, Refshauge KM, et al. Assessment of breast cancer-related arm lymphedema–comparison of physical measurement methods and self-report. Cancer Invest. 2010;28(1):54–62. https://doi.org/10.3109/07357900902918494.
Beelen LM, van Dishoeck AM, Tsangaris E, et al. Patient-reported outcome measures in lymphedema: a systematic review and COSMIN analysis. Ann Surg Oncol. 2021;28(3):1656–68.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Online). 2021;29(372):n71. https://doi.org/10.1136/bmj.n71.
Mokkink LB, Boers M, van der Vleuten CPM, et al. COSMIN risk of bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: a Delphi study. BMC Med Res Methodol. 2020;20(1):293. https://doi.org/10.1186/s12874-020-01179-5.
Mokkink LB, de Vet HCW, Prinsen CAC, et al. COSMIN Risk of Bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1171–9. https://doi.org/10.1007/s11136-017-1765-4.
Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–57. https://doi.org/10.1007/s11136-018-1798-3.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Johansson K, Darkeh MH, Lahtinen T, et al. Two-year follow-up of temporal changes of breast edema after breast cancer treatment with surgery and radiation evaluated by tissue dielectric constant (TDC). Eur J Lymphology Relat Probl. 2015;27(73):15–21.
Johansson K, Lathinen T, Björk-Eriksson T. Breast edema following breast conserving surgery and radiotherapy. Eur J Lymphology Relat Probl. 2014;25(70):1–5.
Garnier M, Champeaux E, Laurent E, et al. High-frequency ultrasound quantification of acute radiation dermatitis: pilot study of patients undergoing radiotherapy for breast cancer. Skin Res Technol. 2017;23(4):602–6. https://doi.org/10.1111/srt.12378.
Wratten C, Kilmurray J, Wright S, et al. A study of high frequency ultrasound to assess cutaneous oedema in conservatively managed breast. Front Radiat Ther Oncol. 2002;37:121–7. https://doi.org/10.1159/000061307.
Wratten C, Kilmurray J, Wright S, et al. Pilot study of high-frequency ultrasound to assess cutaneous oedema in the conservatively managed breast. Int J Cancer. 2000;90(5):295–301.
Wratten CR, O’Brien PC, Hamilton CS, et al. Breast edema in patients undergoing breast-conserving treatment for breast cancer: assessment via high frequency ultrasound. Breast J. 2007;13(3):266–73.
Della Sala SW, Pellegrini M, Bernardi D, et al. Mammographic and ultrasonographic comparison between intraoperative radiotherapy (IORT) and conventional external radiotherapy (RT) in limited-stage breast cancer, conservatively treated. Eur J Radiol. 2006;59(2):222–30.
Tian S, Paster LF, Kim S, et al. Comparison of mammographic changes across three different fractionation schedules for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2016;95(2):597–604. https://doi.org/10.1016/j.ijrobp.2016.01.056.
Carvalho BPSA, Frasson AL, Santos MM, et al. Mammography findings following electron intraoperative radiotherapy or external radiotherapy for breast cancer treatment. Eur J Radiol. 2011;79(2):e7–10. https://doi.org/10.1016/j.ejrad.2009.11.009.
Kuzmiak CM, Zeng D, Cole E, et al. Mammographic findings of partial breast irradiation. Acad Radiol. 2009;16(7):819–25. https://doi.org/10.1016/j.acra.2009.01.021.
Vuorela AL, Harju E, Jakobsson M. Mammographic and palpation findings in the irradiated spared breast. Anticancer Res. 1989;9(4):1217–21.
Eldredge-Hindy H, Gaskins J, Dragun A, et al. Patient-reported outcomes and cosmesis after once-weekly hypofractionated breast irradiation in medically underserved patients. Int J Radiat Oncol Biol Phys. 2020;107(5):934–42. https://doi.org/10.1016/j.ijrobp.2020.04.041.
Degnim AC, Miller J, Hoskin TL, et al. A prospective study of breast lymphedema: frequency, symptoms, and quality of life. Breast Cancer Res Treat. 2012;134(3):915–22. https://doi.org/10.1007/s10549-012-2004-x.
Adriaenssens N, Verbelen H, Lievens P, et al. Lymphedema of the operated and irradiated breast in breast cancer patients following breast conserving surgery and radiotherapy. Lymphology. 2012;45(4):154–64.
Pezner RD, Patterson MP, Robert Hill L, et al. Breast edema in patients treated conservatively for stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 1985;11(10):1765–8. https://doi.org/10.1016/0360-3016(85)90029-x.
Clarke D, Martinez A, Cox RS, et al. Breast edema following staging axillary node dissection in patients with breast carcinoma treated by radical radiotherapy. Cancer. 1982;49(11):2295–9. https://doi.org/10.1002/1097-0142(19820601)49:11%3c2295::Aid-cncr2820491116%3e3.0.Co;2-g.
Rönkä RH, Pamilo MS, Von Smitten KAJ, et al. Breast lymphedema after breast conserving treatment. Acta Oncol. 2004;43(6):551–7. https://doi.org/10.1080/02841860410014867.
Adriaenssens N, Belsack D, Buyl R, et al. Ultrasound elastography as an objective diagnostic measurement tool for lymphoedema of the treated breast in breast cancer patients following breast conserving surgery and radiotherapy. Radiol Oncol. 2012;46(4):284–95. https://doi.org/10.2478/v10019-012-0033-z.
Koban KC, Etzel L, Li Z, et al. Three-dimensional surface imaging in breast cancer: a new tool for clinical studies? Radiat Oncol. 2020;15(1):52. https://doi.org/10.1186/s13014-020-01499-2.
Krishnan L, Stanton AL, Collins CA, et al. Form or function? Part 2. Objective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91(12):2282–7. https://doi.org/10.1002/1097-0142(20010615)91:12%3c2282::AID-CNCR1259%3e3.0.CO;2-0.
Swanick CW, Lei X, Shaitelman SF, et al. Longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Cancer. 2016;122(18):2886–94. https://doi.org/10.1002/cncr.30121.
Chapman BV, Lei X, Patil P, et al. Quantitative 3-dimensional photographic assessment of breast cosmesis after whole breast irradiation for early stage breast cancer: a secondary analysis of a randomized clinical trial. Adv Radiat Oncol. 2020;5(5):824–33. https://doi.org/10.1016/j.adro.2020.04.035.
Pukancsik D, Kelemen P, Újhelyi M, et al. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: an aesthetic and functional prospective cohort study. Eur J Surg Oncol. 2017;43(2):303–10. https://doi.org/10.1016/j.ejso.2016.11.010.
Heil J, Czink E, Golatta M, et al. Change of aesthetic and functional outcome over time and their relationship to quality of life after breast conserving therapy. Eur J Surg Oncol. 2011;37(2):116–21. https://doi.org/10.1016/j.ejso.2010.11.007.
Jankowska-Polańska B, Świątoniowska-Lonc N, Ośmiałowska E, et al. The association between illness acceptance and quality of life in women with breast cancer. Cancer Manag Res. 2020;12:8451–64. https://doi.org/10.2147/cmar.S261624.
Akça M, Ata A, Nayır E, et al. Impact of surgery type on quality of life in breast cancer patients. J Breast Health. 2014;10(4):222–8. https://doi.org/10.5152/tjbh.2014.1919.
Tian Y, Schofield PE, Gough K, et al. Profile and predictors of long-term morbidity in breast cancer survivors. Ann Surg Oncol. 2013;20(11):3453–60. https://doi.org/10.1245/s10434-013-3004-8.
Weng JK, Lei X, Schlembach P, et al. Five-year longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2021;111(2):360–70. https://doi.org/10.1016/j.ijrobp.2021.05.004.
de Oliveira-Junior I, da Silva IA, da Silva FCB, et al. Oncoplastic surgery in breast-conserving treatment: patient profile and impact on quality of life. Breast Care (Basel). 2021;16(3):243–53. https://doi.org/10.1159/000507240.
Jethwa KR, Kahila MM, Mara KC, et al. Patient-reported outcomes of catheter-based accelerated partial breast brachytherapy and whole breast irradiation, a single institution experience. Breast Cancer Res Treat. 2018;169(1):189–96. https://doi.org/10.1007/s10549-018-4665-6.
Teichman SL, Do S, Lum S, et al. Improved long-term patient-reported health and well-being outcomes of early-stage breast cancer treated with partial breast proton therapy. Cancer Med. 2018;7(12):6064–76. https://doi.org/10.1002/cam4.1881.
Ojala K, Meretoja TJ, Leidenius MH. Aesthetic and functional outcome after breast conserving surgery - comparison between conventional and oncoplastic resection. Eur J Surg Oncol. 2017;43(4):658–64. https://doi.org/10.1016/j.ejso.2016.11.019.
Johansson K, Jonsson C, Bjork-Eriksson T. Compression treatment of breast edema: a randomized controlled pilot study. Lymphat Res Biol. 2020;18(2):129–35. https://doi.org/10.1089/lrb.2018.0064.
Mayrovitz HN, Yzer JA. Local skin cooling as an aid to the management of patients with breast cancer related lymphedema and fibrosis of the arm or breast. Lymphology. 2017;50(2):56–66.
Collins SC, Bradley NS, Fitzgibbon S, et al. Kinesiology taping for breast lymphoedema after breast cancer treatment: a feasibility randomised controlled trial. Physiother Pract Res. 2018;39:107–16.
Kilbreath SL, Ward LC, Davis GM, et al. Reduction of breast lymphoedema secondary to breast cancer: a randomised controlled exercise trial. Breast Cancer Res Treat. 2020;184(2):459–67.
Ashforth K, Morgner S, VanHoose L. A new treatment for soft tissue fibrosis in the breast. J Lymphoedema. 2011;6(2):42–6.
Jahr S, Schoppe B, Reisshauer A. Effect of treatment with low-intensity and extremely low-frequency electrostatic fields (Deep Oscillation (R)) on breast tissue and pain in patients with secondary breast lymphoedema. J Rehabilit Med. 2008;40(8):645–50. https://doi.org/10.2340/16501977-0225.
Dylke ES, Benincasa Nakagawa H, Lin L, et al. Reliability and diagnostic thresholds for ultrasound measurements of dermal thickness in breast lymphedema. Lymphat Res Biol. 2018;16(3):258–62. https://doi.org/10.1089/lrb.2016.0067.
Verbelen H, De Vrieze T, Van Soom T, et al. Development and clinimetric properties of the Dutch Breast Edema Questionnaire (BrEQ-Dutch version) to diagnose the presence of breast edema in breast cancer patients. Qual Life Res. 2020;29(2):569–78. https://doi.org/10.1007/s11136-019-02337-z.
Riches K. Determining the size of the problem: a validation study to improve the assessment of mid-line breast cancer related lymphoedema [PhD]: University of Nottingham; 2020.
Kilbreath SL, Fearn NR, Dylke ES. Ultrasound: assessment of breast dermal thickness: Reliability, responsiveness to change, and relationship to patient-reported outcomes. Skin Res Technol. 2021;28(1):111–8. https://doi.org/10.1111/srt.13100.
De Vrieze T, Gebruers N, Nevelsteen I, et al. Reliability of the MoistureMeterD compact device and the pitting test to evaluate local tissue water in subjects with breast cancer-related lymphedema. Lymphat Res Biol. 2020;18(2):116–28. https://doi.org/10.1089/lrb.2019.0013.
Moseley A, Piller N. Reliability of bioimpedance spectroscopy and tonometry after breast conserving cancer treatment. Lymphat Res Biol. 2008;6(2):85–7. https://doi.org/10.1089/lrb.2008.1002.
Leusink A, Connell R, Dean SL, et al. A comparison of volume and anthropometric breast measurements using the Crisalix and VECTRA XT 3-dimensional surface imaging systems in women who have undergone breast-conserving surgery. Med Res Arch. 2021 Apr;9(4). https://doi.org/10.18103/mra.v9i4.2395.
Smith C. The development and validation of the Breast Lymphoedema Severity Symptom (BLYSS) questionnaire [PhD]: Curtin University; 2013.
Brandini da Silva FC, Jose da Silva J, Sarri AJ, et al. Comprehensive validation study of quality-of-life questionnaire using objective clinical measures: Breast Cancer Treatment Outcome Scale (BCTOS) Brazilian Portuguese version. Clin Breast Cancer. 2019;19(1):e85–100. https://doi.org/10.1016/j.clbc.2018.10.004.
Feißt M, Heil J, Stolpner I, et al. Psychometric validation of the Breast Cancer Treatment Outcome Scale (BCTOS-12): a prospective cohort study. Arch Gynecol Obstet. 2019;300(6):1679–86. https://doi.org/10.1007/s00404-019-05362-y.
Hennigs A, Heil J, Wagner A, et al. Development and psychometric validation of a shorter version of the Breast Cancer Treatment Outcome Scale (BCTOS-12). Breast. 2018;38:58–65. https://doi.org/10.1016/j.breast.2017.12.002.
Vieira R, Silva F, Silva MES, et al. Translation and cultural adaptation of the Breast Cancer Treatment Outcome Scale (BCTOS) into Brazilian Portuguese. Rev Assoc Med Bras. 2018;64(7):627–34. https://doi.org/10.1590/1806-9282.64.07.627.
Heil J, Holl S, Golatta M, et al. Aesthetic and functional results after breast conserving surgery as correlates of quality of life measured by a German version of the Breast Cancer Treatment Outcome Scale (BCTOS). Breast. 2010;19(6):470–4. https://doi.org/10.1016/j.breast.2010.05.004.
Struik GM, de Jongh FW, Birnie E, et al. Development and psychometric evaluation of a Dutch-translated shorter Breast Cancer Treatment Outcome Scale (Dutch BCTOS-13). J Patient Rep Outcomes. 2018;2(1):1–10. https://doi.org/10.1186/s41687-018-0085-y.
Stanton AL, Krishnan L, Collins CA. Form or function Part 1 Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91(12):2273–81. https://doi.org/10.1002/1097-0142(20010615)91:12%3c2273::AID-CNCR1258%3e3.0.CO;2-1.
Kerrigan CB, Ahern TP, Brennan SK, et al. Ultrasound for the objective measurement of breast lymphedema. J Ultrasound Med. 2021. https://doi.org/10.1002/jum.15881.
Heydon-White A, Suami H, Boyages J, et al. Assessing breast lymphoedema following breast cancer treatment using indocyanine green lymphography. Breast Cancer Res Treat. 2020;181(3):635–44. https://doi.org/10.1007/s10549-020-05661-y.
Ward LC, Degnim AC, Dylke ES, et al. Bioimpedance spectroscopy of the breast. Lymphat Res Biol. 2020;18(5):448–54. https://doi.org/10.1089/lrb.2019.0087.
Ridner SH, Deng J, Doersam JK, et al. Lymphedema Symptom Intensity and Distress Surveys-Truncal and Head and Neck, Version 2.0. Lymphat Res Biol. 2021;19(3):240–8. https://doi.org/10.1089/lrb.2020.0071.
Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–68. https://doi.org/10.1200/jco.1996.14.10.2756.
Levenhagen K, Davies C, Perdomo M, et al. Diagnosis of upper-quadrant lymphedema secondary to cancer: clinical practice guideline from the oncology section of APTA. Rehabil Oncol. 2017;35(3):E1–18. https://doi.org/10.1097/01.REO.0000000000000073.
Kelemen G, Varga Z, Lázár G, et al. Cosmetic outcome 1–5 years after breast conservative surgery, irradiation and systemic therapy. Pathol Oncol Res. 2012;18(2):421–7. https://doi.org/10.1007/s12253-011-9462-z.
Ridner SH, Montgomery LD, Hepworth JT, et al. Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology. 2007;40(1):35–46.
Ulger H, Erdogan N, Kumanlioglu S, et al. Effect of age, breast size, menopausal and hormonal status on mammographic skin thickness. Skin Res Technol. 2003;9(3):284–9. https://doi.org/10.1034/j.1600-0846.2003.00027.x.
Gutierrez R, Horst KC, Dirbas FM, et al. Breast imaging following breast conservation therapy. In: Dirbas F, Scott-Conner C, editors. Breast Surgical Techniques and Interdisciplinary Management. New York, NY: Springer New York; 2011. p. 975–995.
Qiao Q, Zhou G, Ling Y. Breast volume measurement in young Chinese women and clinical applications. Aesthetic Plast Surg. 1997;21(5):362–8. https://doi.org/10.1007/s002669900139.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported the Joyce Anderson and Betty Schofield Grant – Awarded to KS and Westmead Breast Cancer Institute. NF received a PhD scholarship from this grant.
Author information
Authors and Affiliations
Contributions
All authors contributed to the systematic review conception and design. Titles, abstract and full paper review, data extraction and analysis were performed by NF, SK and CL. SK, ED and KS are PhD supervisors of NF. The first draft of the manuscript was written by NF, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fearn, N., Llanos, C., Dylke, E. et al. Quantification of breast lymphoedema following conservative breast cancer treatment: a systematic review. J Cancer Surviv 17, 1669–1687 (2023). https://doi.org/10.1007/s11764-022-01278-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-022-01278-w