Abstract
Community-acquired pneumonia (CAP) is a common illness that can lead to mortality. β-lactams are ineffective against atypical pathogen including Mycoplasma pneumoniae. We used molecular examinations to develop a decision tree to predict atypical pathogens with CAP and to examine the prevalence of macrolide resistance in Mycoplasma pneumoniae. We conducted a prospective observational study of patients aged ≥ 18 years who had fever and respiratory symptoms and were diagnosed with CAP in one of two community hospitals between December 2016 and October 2018. We assessed combinations of clinical variables that best predicted atypical pathogens with CAP by classification and regression tree (CART) analysis. Pneumonia was defined as respiratory symptoms and new infiltration recognized on chest X-ray or chest computed tomography. We analyzed 47 patients (21 females, 44.7%, mean age: 47.6 years). Atypical pathogens were detected in 15 patients (31.9%; 12 Mycoplasma pneumoniae, 3 Chlamydophila pneumoniae). Ten patients carried macrolide resistant Mycoplasma pneumoniae (macrolide resistant rate 83.3%). CART analysis suggested that factors associated with presence of atypical pathogens were absence of crackles, age < 45 years, and LD ≥ 183 U/L (sensitivity 86.7% [59.5, 98.3], specificity 96.9% [83.8, 99.9]). ur simple clinical decision rules can be used to identify primary care patients with CAP that are at risk for atypical pathogens. Further research is needed to validate its usefulness in various populations.
Trial registration Clinical Trial (UMIN trial ID: UMIN000035346).
Similar content being viewed by others
Introduction
Community-acquired pneumonia (CAP) can lead to mortality [1,2,3]. Atypical pathogens, such as Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila, are recognized as important causes of CAP [4].
Mycoplasma pneumoniae is one of the most common pathogens in CAP [5]. β-lactams, which are commonly-used antimicrobials, are ineffective against it [6]. According to the Cochrane review in 2012, no clinical symptoms or signs are especially useful for reliable diagnosis of CAP with atypical pathogens including Mycoplasma pneumoniae [7]. Diagnostic scoring criteria for consideration of atypical pathogen infections among adult patients with pneumonia were recently published by the Japanese Respiratory Society (JRS), and are now widely used in Japan [8]. Unreliable testing methods were used in the validation study, however, so there has been a need for a more accurate diagnostic method. Where it is available, the molecular method has become an option as a reference test to identify multiple respiratory pathogens. To rule out the diagnosis of atypical pathogens, more reliable criteria are needed. We developed a decision tree to address this gap. To improve the diagnosis of atypical pathogens in patients with pneumonia, we employed several molecular methods.
This study was conducted to develop a decision tree to predict atypical pathogens with CAP confirmed by molecular examinations.
Materials and methods
Subjects
Based on studies of fever in the elderly and on a study of influenza, we recruited patients who were febrile (1 °C higher than their baseline body temperature, or > 37 °C), and who were coughing for at least 3 days [9,10,11]. They were aged ≥ 18 years and were diagnosed with upper respiratory tract infection (URTI) in one of two community hospitals between December 2016 and October 2018. This study focuses on patients with community-acquired pneumonia and was conducted as a part of our prospective observational research investigating the characteristics of atypical pathogen infections [12, 13].
The study sites were the Tone Chuo Hospital (TCH, 253 beds) and the Akashi Medical Center (AMC, 382 beds), both local medical support centers located in Japan with emergency medical care centers and primary care practices. Excluded from this study were patients without informed consent, those with unstable physical conditions (e.g. shock, coma or impaired consciousness), those for whom sample collections were unable to be performed safely, those with history of multiple exacerbations of chronic pulmonary disease, apparent history or presence of dysphagia, presence of obstructive pneumonia, lung abscess, empyema, healthcare-associated pneumonia, or hospital-onset pneumonia referred from other facilities, tuberculosis, nontuberculous mycobacterium lung infections, lung mycosis, sinusitis, or tonsillitis, and patients with a recent history of fever or cough lasting more than 21 days. The patients who took antibiotics at home were not excluded from the study to promote generalizability.
Outcome measures
The primary outcome was CAP with atypical pathogens. Pneumonia was defined as respiratory symptoms and new infiltration that could be recognized on chest X-ray or chest computed tomography [14]. Early in the course of infection, chest CT can sometimes aid in the detection of CAP when chest radiographies are normal [15, 16]. All images were reviewed by a board-certified pulmonary physician (N.I.) for the determination of the final diagnosis. Nasopharyngeal or pharyngeal samples were obtained from all patients at the time of enrollment. Detection of atypical pathogens was made using FilmArray system (Biomérieux, USA) and the FilmArray Respiratory Panel tests for a comprehensive panel of 20 respiratory viruses and bacteria [17]. Analyses of macrolide resistance were performed by GENECUBE Mycoplasma system (TOYOBO, Co., Ltd., Osaka, Japan) [18], because it uses pharyngeal samples and has a higher M. pneumoniae detection rate than nasopharyngeal samples used in FilmArray system [19]. We collected demographic and clinical data on the age, gender, visiting month, comorbidities, history of close contact with confirmed atypical pathogen infections, history of preceding antimicrobial use, history of signs and symptoms (rhinorrhea, sputum, severe cough, sore throat, myalgia, arthralgia, diarrhea, and rash), duration of symptoms at the time of clinical visits, findings of chest auscultation, laboratory findings (white blood cell [WBC] count and C-reactive protein [CRP] levels), CURB-65 score, A-DROP score, and presence of pneumonia [20, 21]. Severe cough was defined as cough with vomiting, or that disturbed sleep, or was persistent [22]. If sputum was available, a quantitative culture was obtained. We used the IFCC-recommended method for lactate dehydrogenase (LD) measurement to reduce fluctuation. If necessary, the physician performed antigen testing (influenza antigen testing, pneumococcal urinary antigen testing, legionella urinary antigen testing, Mycoplasma pneumoniae antigen testing), or loop-mediated isothermal amplification method of sputum sample for the detection of Legionella pneumophila.
The study design was registered as a University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) Clinical Trial (UMIN trial ID: UMIN000035346) on 22 December 2018 (UMIN-CTR URL: http://www.umin.ac.jp/ctr/index.htm). This study was approved by the Akashi Medical Center Research Ethics Committee.
Statistical analyses
To determine the best prediction model for atypical pathogens in CAP patients, we performed classification and regression tree (CART) analysis [23]. In CART analysis, we classified prognostic groups according to the interaction between variables and we decided the cutoff point in each variable. A receiver-operating characteristics (ROC) curve was used to evaluate the sensitivity, specificity, and correct diagnosis rate of the scores for atypical pathogens by JRS guidelines, with the area under the curve (AUC) indicating its discriminatory ability. For analysis of patient characteristics, we used Fisher’s exact test for categorical variables and performed Student’s t test for continuous variables. We also evaluated the utility of the scores for atypical pathogens by the published JRS guidelines in our study population [8]. Scores were determined by the following items: (i) age < 60 years old; (ii) absence of, or only minor underlying diseases; (iii) stubborn cough; (iv) negative or scant chest auscultatory findings; (v) no sputum, or no identified etiological agent by rapid diagnosis; and (vi) white blood cell count < 10,000/μL. The JRS scoring criteria without laboratory tests consisted of items i–v, and a score ≥ 3 were considered to be indicative of an atypical pathogen pneumonia. The scoring criteria with laboratory tests consisted of items i–vi, and a score ≥ 4 was considered indicative of an atypical pathogen infection. All statistical analyses were performed using JMP Pro 11.2.1 software program (SAS Institute Inc., Cary, NC, USA).
Results
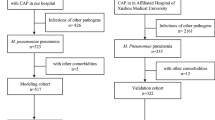
Figure 1 shows the flow of participants, 51 patients were assessed for eligibility. We excluded patients with chronic symptoms (n = 3) and one patient without data on outcome measures (n = 1). The final study population was 47 patients, including 21 females (44.7%). The mean age of the patients was 47.6 (SD 20.1) years old. Besides chest X-ray, chest computer tomography (CT) scan was performed for 19 patients. The most frequent chest CT finding was consolidation, which was found in 15 patients. Four patients were diagnosed with CAP solely by chest CT scan findings. Comorbidities were as follows: chronic heart failure (n = 1, 2.1%), chronic kidney disease (n = 2, 4.3%), chronic liver disease (n = 3, 6.4%), and diabetes mellitus (n = 7, 14.9%). Two patients were immobile. Among all patients, 40 patients (85.1%) reported sputum, 31 patients (66.0%) reported malaise, 24 patients (51.1%) presented with headache, and 24 patients (51.1%) presented with heat sensation. Mean CURB-65 score was 0.4 (SD 0.7) and mean A-DROP score was 0.3 (SD 0.6) (Table 1).
Eighteen patients were admitted on the day of hospital visit. Patients with atypical pathogens were younger than the patients without CAP. Crackles were not found in any patients with atypical pathogen. CURB-65 score, A-DROP score and admission rate were low in patients with atypical pathogen.
Atypical pathogens were found in 15 patients (32%), which included 12 patients with Mycoplasma pneumoniae, including 10 with macrolide resistance, and three patients with Chlamydophila pneumoniae. Macrolide resistance rate among patients with Mycoplasma pneumoniae was 83% (Table 2).
Among these 15 patients, one patient had both atypical pathogen and viral infection (C. pneumoniae with human rhinovirus). Viral infections without accompanying atypical pathogen infections were found in five patients (10.6%). Adenovirus, Bordetella pertussis and Influenza were not detected. Legionella pneumophila was found in a patient’s sputum by loop-mediated isothermal amplification method. Streptococcus pneumoniae were yielded from the sputum cultures of two patients.
Figure 2 shows the decision tree for the presence of atypical pathogens. Among patients with no crackles, those < 45 years of age and those with LD > 183 U/L, 13 out of 14 patients had atypical pathogens. Patients with LD > 183 numbered fourteen in the “all patients” group and just one in the “negative patients” group. The decision tree discriminated atypical pathogens with sensitivity 86.7% (95% CI 0.60–0.98), specificity 96.9% (95% CI 0.84–1.00) and correct diagnosis rate was 93.6% (95% CI 68.0–100%) (Tables 3, 4 and 5).
Using the JRS atypical pathogen diagnostic scoring criteria, 30 (63.8%; 30/47) met the score (≥ 3) for the criteria without laboratory tests, and 19 (45.2%; 19/42) met the score (≥ 4) for the criteria with laboratory tests. The JRS criteria without laboratory tests discriminated atypical pathogens with AUROCC of 0.79, sensitivity 100% (95% CI 69.8–100%), specificity of 53.1% (95% CI 34.7–70.9%) and correct diagnosis rate was 68.1% (95% CI 52.9–80.9%). The JRS criteria with laboratory tests discriminated atypical pathogens with AUROCC of 0.87, sensitivity 100% (95% CI 61.5–100%), specificity of 74.2% (95% CI 55.4–88.1%), and correct diagnosis rate was 81.0% (95% CI 65.9–91.4%) (Tables 3, 4 and 5, Fig. 3).
ROC analysis of decision tree to differentiate the presence of atypical pathogens based on the Japanese guidelines. The decision tree discriminated atypical pathogens with ROC area of 0.87, sensitivity 100%, and specificity 74.2% for the criteria with laboratory tests and ROC area of 0.79, sensitivity 100%, and specificity 53.1% for the criteria without laboratory tests. ROC Receiver-operating curve
Discussion
In summary, to identify primary care patients with CAP that may be at risk for atypical pathogens, our decision tree uses three items: absence of crackles, age < 45 years and LD > 183 U/L. The clinical decision rules can identify primary care patients with CAP at risk for atypical pathogens with high yield (sensitivity 86.7%, specificity 96.9%). It is necessary to compare the diagnostic performance of the JRS criteria in the current study with that of the JRS criteria in previous studies (sensitivity 70.4% and specificity 91.8% in the original study, and sensitivity 77.0% and specificity 93.0% in the validation study) [8, 24]. Ishida et al. validated JRS scoring criteria retrospectively and included patients with Mycoplasma pneumoniae pneumonia, Chlamydophila pneumoniae pneumonia, pneumococcal pneumonia, and Hemophilus influenzae pneumonia. They omitted patients with viral pneumonia, which accounts for approximately 10% of the patients in our study population [8]. We performed validation of JRS scoring criteria of our study participants and the sensitivity and specificity for atypical pathogen infections were 100% and 74.2%, respectively, for the criteria with laboratory tests. Higher detection rate of the pathogens, including viruses by molecular methods would explain the higher sensitivity and lower specificity in our study participants. JRS scoring criteria may have the potential to be utilized to rule out the atypical pathogens with CAP.
Higher detection rate of the pathogens is also required to maintain the diagnostic accuracy in clinical prediction rules because reliable data could not be obtained by using a standard method that is imperfect [25]. Lui et al. assessed CAP-hospitalized patients in a prospective observational study using cultures, antigen testing and paired serology [4]. They could not provide a cutoff point with reasonable sensitivity and specificity to discriminate patients with pneumonia caused by atypical pathogens from patients with bacterial pneumonia. Causal organisms were identified in only 39.2% of their patients [4]. In the present prospective study, 23 out of 47 patients (48.9%) were positive for atypical pathogens. We used a molecular method to identify the pathogens of CAP, a more sensitive method for detection of pathogens than conventional methods. Jain et al. reported that a pathogen was detected in only 38% of patients among adults with radiographic evidence of pneumonia in a prospective population-based surveillance study, although the study did not address any clinical prediction rules [26]. Their study used culture, serologic testing, and antigen detection combined with molecular testing [26]. Most of their specimens, except for blood cultures, were taken after the administration of antimicrobials. In contrast, 34% of participants reported preceding antimicrobial use, which might explain the high-pathogen detection rate in our study.
One of the items used in the present study, age < 45 years, is consistent with previous reports on a clinical prediction rule for atypical pathogens with CAP. [4, 27] Lui et al. developed a prediction rule to discriminate CAP caused by atypical pathogens composed of age < 65 years, female gender, fever ≥ 38.0 °C, respiratory rate < 25/min, pulse rate < 100/min, serum sodium > 130 mmol/L, leucocyte count < 11,000/µL and Hb < 11 g/dL (sensitivity 54.0% and specificity 80.0%) [4]. Their study was designed for hospitalized patients, and a majority of atypical pathogen infections were elderly patients (63.4%) with comorbidities (41.8%) [4]. Older patients are at risk of early mortality, and therefore require hospitalization [28]. In our study, patients were younger and had fewer comorbidities than those in Lui’s study, so our prediction model might be better suited to primary care settings, including outpatients. The prediction model to discriminate CAP caused by Mycoplasma pneumoniae reported by Liu et al. included the characteristics of being < 45 years of age and not coexisting diseases (sensitivity 54.9%, specificity 58%) [27]. The study precluded chlamydophilial infections. It also lacked data on LD, which played a role in the items for discriminating atypical pathogens with CAP in our study.
Macrolide resistance in Mycoplasma pneumoniae being an emerging worldwide problem is also of great importance [29]. Patients with macrolide-resistant Mycoplasma pneumonia have presented prolonged fever and cough with high prevalence of extrapulmonary complications, sometimes resulting in life-threatening infection [30,31,32]. Mutation analysis with molecular methods can reliably determine the presence of macrolide resistance [30, 33]. Among CAP patients, the reported macrolide resistance rate has been reported as 88.3% in China, 70.3% in Korea, 49.4% in Japan, 20% in Italy, 10% in the United States and 3.1% in Germany [34,35,36,37,38,39]. In the present study, macrolide resistance rate was as high as 83.3% among atypical pathogens with CAP. Regional differences in macrolide resistance rate have also been reported in Japan, ranging in prevalence between 50 and 93% [40]. Akashi et al. reported that preceding macrolide use was a risk factor for macrolide resistance [33], although this was uncommon (< 10%) among our patients. The high resistance rates in our study might be associated with regional factors, such as previous excessive use of macrolides and lack of tight control of antimicrobial drug prescriptions. Further adequately sized studies should aim to determine the reason for the high macrolide resistance rate among patients with CAP.
Several limitations associated with the present study warrant mention. First, participants were recruited from just two institutions and a modest number of patients, so validation in future studies is required. Second, we did not include Legionella pneumophila in the respiratory panel tests we used, and one patient with Legionella pneumophila could not therefore be included in the atypical pathogen group. Third, our study excluded patients with critical conditions (shock, coma or impaired consciousness) and some of these patients might have had higher likelihood of pneumonia due to typical pathogens such as Streptococcus pneumoniae. Fourth, we used upper respiratory tract samples for detection of pathogens instead of lower respiratory tract samples (e.g., sputum and bronchial lavage fluid), but collecting and testing of upper respiratory tract samples is a feasible way to increase overall testing rate in office-based settings. Fifth, the decision tree is based on a nonobjective clinical criterion (the presence or absence of crackles). The Japanese Respiratory Society guidelines also use chest auscultatory findings. Moreover, crackles have been reported to have fair to moderate inter-observer agreement (Fleiss’ kappa/intraclass correlation coefficient = 0.4–0.6) to diagnose CAP [41]. Our very simple three-item clinical decision criteria can predict atypical pathogens with CAP, and we suggest it may be used easily in the clinical practices, especially in primary care.
Conclusions
This is the first prospective multicenter study to develop a decision tree to predict atypical pathogens with CAP confirmed by a molecular method. After wider validation in larger studies, our simple clinical decision rules could be useful in identifying primary care patients with CAP that are at risk for atypical pathogens.
Availability of data and materials
Derived data supporting the findings of this study are available from the corresponding author [NI] on request.
Code availability
Not applicable.
References
Almirall J, Bolibar I, Vidal J, Sauca G, Coll P, Niklasson B, Bartolome M, Balanzo X (2000) Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J 15(4):757–763
Yende S, Alvarez K, Loehr L, Folsom AR, Newman AB, Weissfeld LA, Wunderink RG, Kritchevsky SB, Mukamal KJ, London SJ, Harris TB, Bauer DC, Angus DC (2013) Epidemiology and long-term clinical and biologic risk factors for pneumonia in community-dwelling older Americans: analysis of three cohorts. Chest 144(3):1008–1017. https://doi.org/10.1378/chest.12-28181695464
Shephard A, Smith G, Aspley S, Schachtel BP (2015) Randomised, double-blind, placebo-controlled studies on flurbiprofen 8.75 mg lozenges in patients with/without group A or C streptococcal throat infection, with an assessment of clinicians’ prediction of “strep throat.” Int J Clin Pract 69(1):59–71. https://doi.org/10.1111/ijcp.12536
Lui G, Ip M, Lee N, Rainer TH, Man SY, Cockram CS, Antonio GE, Ng MH, Chan MH, Chau SS, Mak P, Chan PK, Ahuja AT, Sung JJ, Hui DS (2009) Role of “atypical pathogens” among adult hospitalized patients with community-acquired pneumonia. Respirology 14(8):1098–1105. https://doi.org/10.1111/j.1440-1843.2009.01637.x
Bamba M, Jozaki K, Sugaya N, Tamai S, Ishihara J, Kori T, Shiro H, Takeuchi Y, Cho H, Nakao A, Okano Y, Kimura K, Komiyama O, Nonoyama M, Kobayashi I, Kato T, Sunakawa K (2006) Prospective surveillance for atypical pathogens in children with community-acquired pneumonia in Japan. J Infect Chemother 12(1):36–41. https://doi.org/10.1007/s10156-005-0422-y
Bebear C, Pereyre S, Peuchant O (2011) Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol 6(4):423–431. https://doi.org/10.2217/fmb.11.18
Wang K, Gill P, Perera R, Thomson A, Mant D, Harnden A (2012) Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev 10:Cd009175. https://doi.org/10.1002/14651858.CD009175.pub2
Ishida T, Miyashita N, Nakahama C (2007) Clinical differentiation of atypical pneumonia using Japanese guidelines. Respirology 12(1):104–110. https://doi.org/10.1111/j.1440-1843.2006.00927.x
Darowski A, Weinberg JR, Guz A (1991) Normal rectal, auditory canal, sublingual and axillary temperatures in elderly afebrile patients in a warm environment. Age Ageing 20(2):113–119. https://doi.org/10.1093/ageing/20.2.113
Norman DC (2000) Fever in the elderly. Clin Infect Dis 31(1):148–151. https://doi.org/10.1086/313896
Kohno S, Yen MY, Cheong HJ, Hirotsu N, Ishida T, Kadota J, Mizuguchi M, Kida H, Shimada J (2011) Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother 55(11):5267–5276. https://doi.org/10.1128/aac.00360-11
Suzuki S, Ishimaru N, Akashi Y, Takeuchi Y, Ueda A, Ushiki A, Kinami S, Suzuki H, Tokuda Y, Maeno T (2020) Physicians’ prediction for the assessment of atypical pathogens in respiratory tract infections. J Gen Fam Med 21(6):226–234. https://doi.org/10.1002/jgf2.350
Ishimaru N, Suzuki S, Shimokawa T, Akashi Y, Takeuchi Y, Ueda A, Kinami S, Suzuki H, Tokuda Y, Maeno T (2020) Heckerling’s criteria to distinguish community-acquired pneumonia in primary care settings: observational validation study in Japan. Asia Pac Fam Med 18(2)
Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, Goto Y, Fukui Y, Iwaki M, Okumura J, Yamaguchi I, Yagi T, Tanikawa Y, Sugino Y, Shindoh J, Ogasawara T, Nomura F, Saka H, Yamamoto M, Taniguchi H, Suzuki R, Saito H, Kawamura T, Hasegawa Y (2013) Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med 188(8):985–995. https://doi.org/10.1164/rccm.201301-0079OC
Upchurch CP, Grijalva CG, Wunderink RG, Williams DJ, Waterer GW, Anderson EJ, Zhu Y, Hart EM, Carroll F, Bramley AM, Jain S, Edwards KM, Self WH (2018) Community-acquired pneumonia visualized on CT scans but not chest radiographs: pathogens, severity, and clinical outcomes. Chest 153(3):601–610. https://doi.org/10.1016/j.chest.2017.07.035
Feldman C (2001) Pneumonia in the elderly. Med Clin N Am 85(6):1441–1459. https://doi.org/10.1016/s0025-7125(05)70390-4
Poritz MA, Blaschke AJ, Byington CL, Meyers L, Nilsson K, Jones DE, Thatcher SA, Robbins T, Lingenfelter B, Amiott E, Herbener A, Daly J, Dobrowolski SF, Teng DH, Ririe KM (2011) FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS ONE 6(10):e26047. https://doi.org/10.1371/journal.pone.0026047
Ito Y, Iwashima S, Hayano S, Nishio T, Shiozawa R, Yata S, Kubota T, Kubota A, Uemura K (2018) Rapid detection of the macrolide sensitivity of pneumonia-causing Mycoplasma pneumoniae using quenching probe polymerase chain reaction (GENECUBE((R))). Mol Diagn Ther 22(6):737–747. https://doi.org/10.1007/s40291-018-0360-x
Gnarpe J, Lundbäck A, Gnarpe H, Sundelöf B (1997) Comparison of nasopharyngeal and throat swabs for the detection of Chlamydia pneumoniae and Mycoplasmapneumoniae by polymerase chain reaction. Scand J Infect Dis Suppl 104:11–12
Miyashita N, Matsushima T, Oka M, Japanese Respiratory S (2006) The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med 45(7):419–428. https://doi.org/10.2169/internalmedicine.45.1691
Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT (2003) Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58(5):377–382. https://doi.org/10.1136/thorax.58.5.377
Hayashi D, Akashi Y, Suzuki H, Shiigai M, Kanemoto K, Notake S, Ishiodori T, Ishikawa H, Imai H (2018) Implementation of point-of-care molecular diagnostics for Mycoplasma pneumoniae ensures the correct antimicrobial prescription for pediatric pneumonia patients. Tohoku J Exp Med 246(4):225–231. https://doi.org/10.1620/tjem.246.225
Breiman L (2017) Classification and regression trees. Routledge
Ishida T, Hashimoto T, Arita M, Kaneshiro E, Osawa M, Tachibana H, Nishioka N, Watanabe K (2002) Evaluation of community-acquired pneumonia guidelines of Japanese Respiratory Society: differentiation of atypical pneumonia and bacterial pneumonia. Nihon Kokyuki Gakkai Zasshi 40(12):929–935
Reitsma JB, Rutjes AW, Khan KS, Coomarasamy A, Bossuyt PM (2009) A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 62(8):797–806. https://doi.org/10.1016/j.jclinepi.2009.02.005
Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L, Team CES (2015) Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373(5):415–427. https://doi.org/10.1056/NEJMoa1500245
Liu YF, Gao Y, Chen MF, Cao B, Yang XH, Wei L (2013) Etiological analysis and predictive diagnostic model building of community-acquired pneumonia in adult outpatients in Beijing, China. BMC Infect Dis 13:309. https://doi.org/10.1186/1471-2334-13-309
Garcia-Vidal C, Fernández-Sabé N, Carratalà J, Díaz V, Verdaguer R, Dorca J, Manresa F, Gudiol F (2008) Early mortality in patients with community-acquired pneumonia: causes and risk factors. Eur Respir J 32(3):733–739. https://doi.org/10.1183/09031936.00128107
Tanaka T, Oishi T, Miyata I, Wakabayashi S, Kono M, Ono S, Kato A, Fukuda Y, Saito A, Kondo E, Teranishi H, Tanaka Y, Wakabayashi T, Akaike H, Ogita S, Ohno N, Nakano T, Terada K, Ouchi K (2017) Macrolide-resistant Mycoplasma pneumoniae infection, Japan, 2008–2015. Emerg Infect Dis 23(10):1703–1706. https://doi.org/10.3201/eid2310.170106
Miyashita N, Akaike H, Teranishi H, Ouchi K, Okimoto N (2013) Macrolide-resistant Mycoplasma pneumoniae pneumonia in adolescents and adults: clinical findings, drug susceptibility, and therapeutic efficacy. Antimicrob Agents Chemother 57(10):5181–5185. https://doi.org/10.1128/AAC.00737-13
Matsumoto M, Nagaoka K, Suzuki M, Konno S, Takahashi K, Takashina T, Ishiguro N, Nishimura M (2019) An adult case of severe life-threatening Mycoplasma pneumoniae pneumonia due to a macrolide-resistant strain, Japan: a case report. BMC Infect Dis 19(1):204–204. https://doi.org/10.1186/s12879-019-3846-1
Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z (2014) More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother 58(2):1034–1038. https://doi.org/10.1128/AAC.01806-13
Akashi Y, Hayashi D, Suzuki H, Shiigai M, Kanemoto K, Notake S, Ishiodori T, Ishikawa H, Imai H (2018) Clinical features and seasonal variations in the prevalence of macrolide-resistant Mycoplasma pneumoniae. J Gen Fam Med 19(6):191–197. https://doi.org/10.1002/jgf2.201
Miyashita N, Kawai Y, Akaike H, Ouchi K, Hayashi T, Kurihara T, Okimoto N, Atypical Pathogen Study G (2012) Macrolide-resistant Mycoplasma pneumoniae in adolescents with community-acquired pneumonia. BMC Infect Dis 12:126–126. https://doi.org/10.1186/1471-2334-12-126
Yoon IA, Hong KB, Lee HJ, Yun KW, Park JY, Choi YH, Kim WS, Lee H, Eun BW, Ahn YM, Cho EY, Cho HJ, Choi EH (2017) Radiologic findings as a determinant and no effect of macrolide resistance on clinical course of Mycoplasma pneumoniae pneumonia. BMC Infect Dis 17(1):402–402. https://doi.org/10.1186/s12879-017-2500-z
Qu J, Chen S, Bao F, Gu L, Cao B (2019) Molecular characterization and analysis of Mycoplasma pneumoniae among patients of all ages with community-acquired pneumonia during an epidemic in China. Int J Infect Dis 83:26–31. https://doi.org/10.1016/j.ijid.2019.03.028
Loconsole D, De Robertis AL, Mallamaci R, Sallustio A, Morea A, Prato R, Quarto M, Martinelli D, Chironna M (2019) First description of macrolide-resistant Mycoplasma pneumoniae in adults with community-acquired pneumonia in Italy. Biomed Res Int 2019:7168949–7168949. https://doi.org/10.1155/2019/7168949
Dumke R, Schnee C, Pletz MW, Rupp J, Jacobs E, Sachse K, Rohde G, Capnetz Study G (2015) Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg Infect Dis 21(3):426–434. https://doi.org/10.3201/eid2103.140927
Diaz MH, Benitez AJ, Winchell JM (2015) Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance from 2006 to 2013. J Clin Microbiol 53(1):124–130. https://doi.org/10.1128/JCM.02597-14
Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, Saito A, Kondo E, Teranishi H, Wakabayashi T, Ogita S, Tanaka T, Kawasaki K, Nakano T, Terada K, Ouchi K (2013) Nationwide surveillance of macrolide-resistant Mycoplasma pneumoniae infection in pediatric patients. Antimicrob Agents Chemother 57(8):4046–4049. https://doi.org/10.1128/AAC.00663-13
Florin TA, Ambroggio L, Brokamp C, Rattan MS, Crotty EJ, Kachelmeyer A, Ruddy RM, Shah SS (2017) Reliability of examination findings in suspected community-acquired pneumonia. Pediatrics 140(3):e20170310. https://doi.org/10.1542/peds.2017-0310
Acknowledgements
We are very grateful to the laboratory staff and physicians of the outpatient clinics at Tone Chuo Hospital and Akashi Medical Center for their significant contributions to this work. We thank Benjamin Phillis of Akashi Medical Center for proofreading and editing the manuscript.
Funding
This study was supported by TOYOBO Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Hiromichi Suzuki received lecture fees and consulting fees from TOYOBO Co., Ltd. The other authors have no conflicts of interest to disclose with respect to this research.
Ethics approval
This study was approved by the Akashi Medical Center Research Ethics Committee.
Consent to participate
Written informed consent was obtained from the patient for participation of this study.
Consent for publication
Written informed consent was obtained from the patient for publication of this research.
Informed consent
Informed consent was obtained from all individual participants included in the study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishimaru, N., Suzuki, S., Shimokawa, T. et al. Predicting Mycoplasma pneumoniae and Chlamydophila pneumoniae in community-acquired pneumonia (CAP) pneumonia: epidemiological study of respiratory tract infection using multiplex PCR assays. Intern Emerg Med 16, 2129–2137 (2021). https://doi.org/10.1007/s11739-021-02744-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-021-02744-6