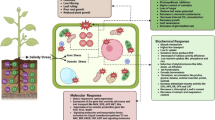
Abstract
Salt stress is abiotic stress that negatively impacts plant growth. Exogenous spermidine (SPD) has been proposed as a priming agent that effectively alleviates various abiotic stresses in plants. To evaluate the alleviation role of exogenous SPD application against salt stress, we studied the physiological and biochemical changes, and the expression of gene encoding antioxidant enzymes and phytohormones in the SPD-treated pecan-grafted seedling. The results revealed that for the seedlings growing under salt stress environment, a drop in the total chlorophyll content and photosynthetic capability was observed. These effects subsided when the seedlings were treated with SPD. Moreover, the malondialdehyde level and Na+/K+ ratio, as well as the damage of the chloroplast ultrastructure in the seedlings, were markedly suppressed after SPD treatment. Compared with the non-SPD-treated counterparts, the exogenous SPD application promoted higher antioxidant enzyme activities and their corresponding gene expression. At the same time, the induction of abscisic acid and ethylene was suppressed. The results indicate that the exogenous SPD plays a vital role in alleviating salt-incurred phytotoxicity and oxidative damage in pecan-grafted seedlings. The remissive role of exogenous SPD is implicated by its interplay with the expression of the antioxidant enzymes and phytohormones in the pecan-grafted seedlings.






Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- CAT:
-
Catalase
- Chl:
-
Chlorophyll
- DAT:
-
Day after treatment
- ETH:
-
Ethylene
- Fv/Fm :
-
Maximum quantum yield of PSII photochemistry
- MDA:
-
Malondialdehyde
- PA:
-
Polyamine
- Pn:
-
Net photosynthetic rate
- POD:
-
Peroxidase
- PVP:
-
Polyvinylpyrrolidone
- SPD:
-
Spermidine
- SPM:
-
Spermine
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
- TBA:
-
Thiobarbituric acid
References
Ahmed MZ, Shimazaki T, Gulzar S, Kikuchi A, Gul B, Khan MA (2013) The influence of genes regulating transmembrane transport of Na+ on the salt resistance of Aeluropus lagopoides. Funct Plant Biol 40:860–871
Antolín MC, Santesteban H, Santa María E, Aguirreolea J, Sánchez-díaz M (2008) Involvement of abscisic acid and polyamines in berry ripening of Vitis vinifera (L.) subjected to water deficit irrigation. Aust J Grape Wine Res 14:123–133
Bleecker AB, Kende H (2000) Ethylene, a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Borzouei A, Jamali SS, Paknejad F (2015) Root characteristics, Na+/K+ ratio and grain yield of seven wheat genotypes under salinity stress. J Sci Tech Greenh Cult 5:165–175
Cao W, Liu J, He X, Mu R, Zhou H, Chen S, Zhang J (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143:707–719
Chen J, Zhu C, Li LP, Sun ZY, Pan XB (2007) Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. J Environ Sci 19:44–49
Childs AC, Mehta DJ, Gerner EW (2003) Polyamine-dependent gene expression. Cell Mol Life Sci 60:1394–1406
Choudhary SP, Kanwar M, Bhardwaj R, Yu JQ, Tran LS (2012) Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 7:e33210
Ding S, Zhang B, Qin F (2015) Arabidopsis RZFP34/CHYR1, a Ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2.6-mediated phosphorylation. Plant Cell 27:3228–3244
Evans PT, Malmberg RL (1989) Do polyamines have roles in plant development? Ann Rev Plant Physiolol 40:235–269
Feng Y, Zhuang B (2017) Flame atomic absorption spectrometry for the determination of potassium and sodium in Yanhuning. Channel Pharm 29:58–60
Gama PBS, Tanaka K, Ahmed Z, Siddig KE (2007) Salinity induced stress effects on biomass, photosynthetic rate, and ROS-scavenging enzymes accumulation of Phaseolus vulgaris L. Acta Hort 746:381–386
Gómez-Pando LR, Álvarez-Castro R, Eguiluz-de la Barra A (2010) Effect of salt stress on peruvian germplasm of Chenopodium quinoa Willd, A promising crop. J Agron Crop Sci 196:391–396
Guo H, Hong C, Chen X, Xu Y, Liu Y, Jiang D, Zheng B (2016) Different growth and physiological responses to cadmium of the three Miscanthus species. PLoS ONE 11:e0153475
Hariadi Y, Marandon K, Tian Y, Jacobsen SE, Shabala S (2011) Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J Exp Bot 62:185–193
Hatami E (2018) Alleviating salt stress in almond rootstocks using of humic acid. Sci Hortic 237:296–302
Hernández JA, Jiménezz MP, Sevilla F (2010) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23:853–862
Hong C, Guo H, Fang J, Ren W, Wang T, Ji MC (2014) Physiological and biochemical responses of Miscanthus sacchariflorus to salt stress. Adv Mater Res 1051:333–340
Hu L, Xiang L, Li S, Zou Z, Hu X (2015) Beneficial role of spermidine in chlorophyll metabolism and D1 protein content in tomato seedlings under salinity-alkalinity stress. Physiol Plant 156:468–477
Huang Y, Liu L, Huang JQ, Wang Z, Chen F, Zhang Q, Zheng B, Chen M (2013) Use of transcriptome sequencing to understand the pistillate flowering in hickory (Carya cathayensis Sarg.). BMC Genom 14:1–18
Jampeetong A, Brix H (2009) Effects of NaCl salinity on growth morphology, photosynthesis and proline accumulation of Salvinia natans. Aquat Bot 91:181–186
Javid MG, Sorooshzadeh A, Moradi F, Modarres SAM, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Aust J Crop Sci 32:726–734
Jungmyung L (2010) Current status of vegetable grafting, diffusion, grafting techniques, automation. Sci Hortic 127:93–105
Katahata S, Naramoto M, Kakubari Y, Mukai Y (2007) Photosynthetic capacity and nitrogen partitioning in foliage of the evergreen shrub Daphniphyllum humile along a natural light gradient. Tree Physiol 27:199–208
Klein A, Hüsselmann L, Keyster M, Ludidi N (2018) Exogenous nitric oxide limits salt-induced oxidative damage in maize by altering superoxide dismutase activity. S Afr J Bot 115:44–49
Kubis J (2005) The effect of exogenous spermidine on superoxide dismutase activity, H2O2 and superoxide radical level in barley leaves under water deficit conditions. Acta Physiol Plant 27:289–295
Li Y, Shi G, Wang H, Zhao J, Yuan Q (2009) Exogenous spermidine can mitigate the poison of cadmium in Nymphoides peltatum. Chin Bull Bot 5:571–577
Li B, Sang T, He L (2013) Exogenous spermidine inhibits ethylene production in leaves of cucumber seedlings under NaCl stress. J Am Soc Hortic Sci 138:108–113
Liu H, Yu B, Zhang W, Liu Y (2005) Effect of osmotic stress on the activity of H+-ATPase and the levels of covalently and noncovalently conjugated polyamines in plasma membrane preparation from wheat seedling roots. Plant Sci 168:1599–1607
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Ma L, Zhang H, Sun L, Jiao YH, Zhang GZ, Miao C, Hao FS (2012a) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Exp Bot 63:305–317
Ma T, Chen HW, Xiong XW (2012b) Effects of stock and scion on graft survival rate and growth of Carya illinoensis. J Northwest For Univ 27:141–143
Mansour MMF (2012) Plasma membrane permeability as an indicator of salt tolerance in plants. Biol Plant Biol Plant 57:1–10
Margulies MM (1962) Effect of chloramphenicol on light dependent development of seedlings of phaseolus vulgaris var. black valentine, with particular reference to development of photosynthetic activity. Plant Physiol 37:473–480
Mehta RA, Cassol T, Li N (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotechnol 20:613–618
Metternicht G, Zinck A (2003) Remote sensing of soil salinity: potentials and constraints. Remote Sens Environ 85:1–20
Minocha R (2014) Polyamines and abiotic stress in plants, a complex relationship. Front Plant Sci 5:175–192
Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA (2008) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20:1708–1724
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
O'Neil CE (2010) Tree nut consumption improves nutrient intake and diet quality in US adults, an analysis of National Health and Nutrition Examination Survey (NHANES) 1999–2004. Asia Pac J Clin Nutr 19:142–150
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants, a review. Ecotoxicol Environ Saf 60:324–349
Petronia C, Danila P, Pasqualina W, Giovanni P, Giuseppina M, Maria GA, Amodio F, Ronan S (2011) Salt-induced accumulation of glycine betaine is inhibited by high light in durum wheat. Funct Plant Biol 38:139–150
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5:1–16. https://doi.org/10.3389/fpls.2014.00154
Roy P, Niyogi K, Sengupta DN, Ghosh B (2005) Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt-sensitive rice cultivars. Plant Sci 168:583–591
Sanchez DH, Cuevas JC, Chiesa MA, Ruiz OA (2005) Free spermidine and spermine content in Lotus glaber under long-term salt stress. Plant Sci 168:541–546
Shi H (2013) Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the Bermudagrass (Cynodon dactylon) response to salt and drought stresses. J Proteome Res 12:4951–4964
Smadar H, Mee YG, Mattoo AK, Kieber JJ (2012) The formation of ACC and competition between polyamines and ethylene for SAM. Annu Plant Rev 44:53–81
Stark F, Pfannstiel J, Klaiber I, Raabe T (2011) Protein kinase CK2 links polyamine metabolism to mapk signalling in drosophila. Cell Signal 23:876–882
Sudha G, Ravishankar GA (2002) Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Organ Cult 71:181–212
Sun J, Zou DT, Luan FS, Zhao HW, Wang JG, Liu HL, Xie DW, Su DQ, Ma J, Liu ZL (2014) Dynamic QTL analysis of the Na+ content, K+ content, and Na+/K+ ratio in rice roots during the field growth under salt stress. Biol Plant 58:689–696
Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53:749–790
Tiwari JK, Munshi AD, Kumar R, Pandey RN, Arora A, Bhat JS, Sureja AK (2010) Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol Plant 32:103–114
Wang R, Hua C, Luo Q, Liu Y (2002) Na+ and K+ accumulation in chloroplasts results in a decrease in net photosynthetic rate in rice leaves under salt stress. Acta Photophysiol Sinica 28:385–390
Wang L, Liu J, Wang W, Sun Y (2016) Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induce stress. Photosynthetica 54:19–27
Wu J, Shu S, Li C (2018) Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol Biochem 128:152–162
Xu Y, Wang J, Shan L, Dong X, Li M (2001) Effect of exogenous polyamines on glycolate oxidase activity and active oxygen species accumulation in wheat seedlings under osmotic stress. Isr J Plant Sci 49:173–178
Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, Liu G, Ren D (2008) Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem 283:26996–27006
Xu Q, Wu J, Cao Y, Yang X, Wang Z, Huang J, Xia G, Zhang Q, Hu Y (2016) Photosynthetic characteristics of leaves and fruits of Hickory (Carya cathayensis Sarg.) and Pecan (Carya illinoensis K.Koch) during fruit development stages. Trees 30:1–12
Yang L, Zhu Y, Hu C (2007) Effects of NaCl stress on polyamine contents in grafted cucumber leaves. Chin J Appl Ecol 18:831–836
Zapata PJ, Serrano M, Pretel MT (2003) Changes in ethylene evolution and polyamine profiles of seedlings of nine cultivars of Lactuca sativa L. in response to salt stress during germination. Plant Sci 164:557–563
Zeng Y, Zhang Y, Xiang J, Wu H, Chen H, Zhang Y (2016) Effects of chilling tolerance induced by spermidine pretreatment on antioxidative activity, endogenous hormones and ultrastructure of indica-japonica hybrid rices seedlings. J Integr Agric 15:295–308
Zhang Z, Qu WP (2003) The experimental guide for plant physiology, 3rd edn. Higher Education Press, Beijing, pp 67–69
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res 97:111–119
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588
Zhao S, Xu C, Zou Q (1994) Improvements of method for measurement of malondialdchyde in plant tissues. Plant Physiol Commun 30:207–210
Zhao F, Song CP, He J, Zhu H (2007) Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol 145:1061–1072
Zhao J, Sun F, Yao X (2012) Effects of salt stress on growth and photosynthetic characteristics of Carya Illinoemis. J Southwest China Norm Univ 37:93–97
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 16:527–533
Acknowledgements
This study was supported by National Key Research and Development Program of China (2018YFD1000600, 2018YFD1000604); Key Project of Zhejiang Provincial Natural Science Foundation (LZ18C160001); Independent Research Project of State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University (ZY20180208, ZY20180308); Key Research and Development Program of Zhejiang Province (2018C02004); National Natural Science Foundation of China (31470683, 31270716 and 31070604); National High Technology Research and Development Program of China (863 Program) (2013AA102605); Open Foundation of State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University (KF201708); Fruit Innovation Team Project of Zhejiang Province (2016C02052-12); Key Agricultural New Varieties Breeding Projects founded by Zhejiang Province Science and Technology Department (2016C02052-13); Open Foundation of First-class Discipline of Forestry, Zhejiang Province (201703); National Undergraduate Innovation and Entrepreneurship Training Project (201610341010); Undergraduate Science and Technology Innovation Plan of Zhejiang Province (2017R412006); Undergraduate Research Training Program in Zhejiang A & F University (KX20180047, KX20180043, KX20180065).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Z., Wang, J., Yan, D. et al. Exogenous spermidine improves salt tolerance of pecan-grafted seedlings via activating antioxidant system and inhibiting the enhancement of Na+/K+ ratio. Acta Physiol Plant 42, 83 (2020). https://doi.org/10.1007/s11738-020-03066-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03066-4