Abstract
The aim of the study was to evaluate the biosynthesis and exudation of 10 low-molecular weight organic acids (LMWOAs) into the rhizosphere with a simultaneous analysis of the acid contents in the roots and leaves of 9 Salix taxa growing on two experimental areas, differing in their concentrations of copper (Cu), lead (Pb) and zinc (Zn) in the soil (Area 1—low, Area 2—high concentration). The obtained results reveal a significant difference in the phytoextraction of the tested Salix taxa for the analysed metals in both areas. The highest contents of Cu, Pb and Zn were observed for all Salix collected from Area 2, especially in S. × smithiana roots (116 ± 8.76, 87.84 ± 7.30 and 203.42 ± 14.62 mg kg−1 DW, respectively). The results obtained in Area 2 also revealed acidification of the rhizosphere and a higher concentration of acids, mainly oxalic, malic, malonic, acetic and citric acids. Contents of oxalic, malic, acetic and citric acids increased in the roots of Salix taxa from Area 2, while in the leaves formic and succinic acids were also present. S. × smithiana was the taxon with the highest concentration of acids in the rhizosphere and roots (73.48 ± 6.77 and 49.79 ± 2.65 μM 100 g−1 DW, respectively), while in leaves a higher content was observed for S. alba and S. viminalis ‘PR’ taxa (78.12 ± 3.95 and 71.12 ± 3.75 μM 100 g−1 DW, respectively).
Similar content being viewed by others
Introduction
Metal uptake by plants is modulated by enhanced production, exudation and accumulation of low-molecular weight organic acids (LMWOAs) upon exposure to trace elements. Copper (Cu) and zinc (Zn) are important as micronutrients that participate in normal plant development. Cu is a component of plastocyanin (PCY), cytochrome oxidase (COX), ascorbate oxides (AAO) and tyrosinase, while Zn is present in superoxide respiratory (SOD) and nitrite reductase development (Puig and Thiele 2002). However, in high concentration it may be phytotoxic and can interfere with enzyme function and as a consequence disturb the metabolism of numerous biomolecules (Van Assche and Clijsters 1990; Liu et al. 2004; Albarracín et al. 2010).
The use of plants for remediation of contaminated soil depends on the phenotype and genotype of the plant, the presence of arbuscular mycorrhizal (AM) fungi (abundant soil micro-organisms, which can improve Zn tolerance and increase plant nutrition), and such an important factor as the interaction between organic matter, the rhizosphere and metals due to solubility and availability of metals (Chen et al. 2003; Vivas et al. 2006). Metals are strongly fixated with organic matter, clays or oxides and thus are not available for the plant (Chen et al. 2003). Exudation of LMWOAs has been shown to be one of the most important factors for mobilization and for increasing the bioavailability of low soluble nutrients [e.g. phosphorus (P), iron (Fe), Zn]. It is also reported that LMWOAs have the ability to detoxify some harmful metals [cadmium (Cd), mercury (Hg), arsenic (As), lead (Pb)] (Jones and Darrah 1994; Neumann and Rőmheld 1999; Dakora and Phillips 2002; Kutrowska and Szelag 2014). Depending on environmental conditions, plant roots exudate different profiles as well as concentrations of LMWOAs into the rhizosphere (Zeng et al. 2008). Exudation of organic molecules by roots is considered as one of the most important strategies of plants to tolerate the presence of trace elements. Organic acids are exudated as anions and this process is balanced by the release of cations (Janicka-Russak et al. 2008). The anions ultimately consume protons, particularly when the substrate pH is low. In this context, it is likely that releasing organic anions, like LMWOAs, from the plant roots is treated as a defence mechanism on exposure to metals (UdDin et al. 2015). LMWOAs are able to exclude these metals by their chelation directly into the rhizosphere and/or in the subsequent step in the apoplastic, thus preventing their entry into the symplast (Nigam et al. 2001; Meier et al. 2012). Although the exudation process is well documented for agricultural plants (Duarte et al. 2011), studies on the interaction between metals, phytoextraction and LMWOAs are limited. Salix taxa were chosen on the basis of their significant traits such as fast growth, easy adaption to new environmental conditions and low ecological requirements, with the exception of water level, necessary in phytoextraction of trace elements (Guidi Nissim and Labrecque 2016).
However, the role of the LMWOAs is not limited to the rhizosphere. The total content of LMWOAs in plant organs is high due to their important role as photosynthetic intermediates and their potential role as a metabolically active solution for osmotic adjustment. Organic acids also participate as key components in the mechanisms that some plants use to cope with metal tolerance, as natural chelators buffering cytosolic excesses of trace elements (Clemens 2001; Martins et al. 2013; Goliński et al. 2015). However, the biosynthesis, accumulation and transport of organic acids become significantly changed in response to species, cultivars, age and growth condition. Literature data suggest that chelation with LMWOAs should be seen as an important defence mechanism to efficiently transport and reduce the toxicity of free metal ions in plants (Wei et al. 2009; Ghnaya et al. 2013). Tiffin (1970) demonstrated that the amount of metal-citrate complexes increases with higher accumulation of Zn in stems and leaves. Similar observations were made by Senden and Wolterbeek (1990), who described the same relation between an increase of Cu content and the amount of metal-citrate complexes in Papyrus stems. In the xylem, it was found that these complexes are efficiently transported to the negatively charged vessel walls by a lower adsorption and a diminution of lateral escape (Tiffin 1970; Senden and Wolterbeek 1990).
The aim of the study was to evaluate the biosynthesis and exudation of 10 LMWOAs into the rhizosphere with a simultaneous analysis of the acid contents in the roots and leaves of 9 Salix taxa growing on two experimental areas with different environmental conditions. To compare the creation of LMWOAs, an analysis of Cu, Pb and Zn contents in leaves and roots was performed.
Materials and methods
Site description
Plants were cultivated in two different experimental areas, differing in the concentration of selected metals in their soil (Table 1).
The experimental areas are situated in the lowland part of Poland and their terrain is flat. The first experimental area (Area 1) is located in the Zielonka Experimental Forest Division (belonging to Poznan University of Life Sciences,). The coordinates of the centre of Area 1 are 52°33′4″N, 17°06′20″E. The second area (Area 2) is near Grodziec Mały Village (the coordinates of the centre of the area are 51°40′48″N, 16°02′41″E). Both areas differ with regard to the type of bedrock (Area 1—outwash sand, Area 2—alluvial clay), type of water regime (Area 1—ground water level at a depth of 11.0 m; Area 2—periodically flooded) and element concentration.
Characteristics of tested Salix taxa
The tested willows were selected from the Willow Collection of the Poznan University of Life Sciences (PULS), located in different parts of Poland (Rutkowski 2013). The collection comprises 150 genotypes (10 species and 17 natural hybrids differentiated by sex and varieties), planted in 2011 randomly with repetition in 34 experimental blocks, in six localizations (one in northern, two in the middle-west and three in the south-western part of Poland; two of them were chosen for this study). The number of blocks in all the localizations was varied from 4 to 8 per localization, depending on the soil homogeneity of each site, but the planting design was identical in every place, so the area of each experimental block and the number of planted cuttings in each block were the same—3 cuttings for each genotype × 150 genotypes for each block.
For the purposes of the present study, specimens representing 9 Salix genotypes were chosen. The following Salix taxa were analysed: S. × smithiana (S 1), S. × rubra 4 (S 30), S. × smithiana 2 (S 44), S. purpurea × triandra × viminalis 2 (S 46), S. × purpurea 10 (S 58), S. cinerea (S 205/189), S. × smithiana (S 207/191), S. alba (S 225) and S. viminalis ‘PR’ (S 305/305). S. × smithiana (S 1) and S. × smithiana (S 207/191) were two separate Salix taxa characterized by different sex (S. × smithiana (S 1) was female, while S. × smithiana (S 207/191) male specimens).
In April 2011, willow cuttings 25–30 cm in length were prepared from a nursery located near Dobrygość village (51°15′11.0″N; 18°09′52.0″E). The cuttings were planted by hand, at a depth of about 20–25 cm, leaving about 5 cm of the cutting above ground. The cuttings were spaced at a distance of 0.5 m in each row and 1.5 m was left between the rows of planted willows. The estimated density was 12,500 plants per ha. Throughout the cultivation period, all plots were weeded by power scythe. No fertilizer was used on the research areas, none of the plots were watered and no pesticides or fungicides were applied.
Sample collection
For analysis of metals and LMWOAs, 21–35 g of leaves from the 2-year-old plants (June 2013) were collected from the central parts of the plant shoots. Roots with a diameter greater than 0.5 cm and the rhizosphere around them were also collected. Five Haplic Fluvisol (eutric) soil samples were taken from the Ap soil horizon, using a soil auger (4-cm-diameter pipe) around (0.5 m) each studied plant.
Metal analysis in soil and plant organs
Sampling
The collected leaves and roots were washed with deionized water (Milli-Q Advantage A10 Water Purification Systems, Merck Millipore) to determine the total content of elements in these organs so as to remove ions absorbed on the leaf surface or plant root. Both leaves and roots were dried in an electric oven for 98 h at 105 ± 5 °C and ground in a laboratory Boll Mill PM 200 (Retsch GmbH, Haan, Germany) for 3 min to obtain a powder fraction. Three representative subsamples of 0.5000 ± 0.0001 g each were digested in a CEM Mars 5 Xpress (CEM, Matthews, NC) microwave mineralization system, using 6 mL of concentrated (65%) HNO3 and 2 mL of 30% H2O2 (Sigma–Aldrich). Digestion, performed according to our own procedure, consisted of three stages: 1st—temperature 120 °C, 6 min at power 800 W; 2nd—temperature 180 °C, 8 min at power 1200 W and 3rd—temperature 200 °C, 10 min at power 1600 W. After digestion, the samples were filtered using 45-mm filters (Qualitative Filter Papers Whatman, Grade 595: 4–7 µm) and the obtained supernatant was diluted with deionized water (Merck, Darmstadt, Germany) to a final volume of 50 mL.
Soil samples (of approximately 15 g each) were dried in an even layer on Petri dishes within 96 h, transferred to an agate mortar and ground. A Vibratory Sieve Shaker AS 200 digit (Retsch, GmbH, Haan, Germany) was used to remove of all soil skeleton particles. For the analysis only fine particles (d < 2 mm) were used. Soil samples were digested in the same way as plant organs but after a macerization process in 65% HNO3 within 24 h. The obtained supernatants after filtration were also diluted with deionized water to a final volume of 50 mL, which allowed the “pseudo total” concentrations of Cu, Pb and Zn to be determined. Additionally, soil extraction with 1 mol L−1 of HCl (Sigma–Aldrich) was performed to ascertain the concentration of bioavailable metals (Mocek and Drzymała 2010).
Cu, Pb and Zn content analysis
Analysis of Cu, Pb and Zn contents in Salix shoots and leaves was carried out using flame atomic absorption spectrometry (FAAS) using an AA Duo—AA280FS/AA280Z spectrometer (Agilent Technologies, Mulgrave, Victoria, Australia). Calibration curves were prepared for four replicates per each metal concentration out of a stock solution of 1000 mg dm−3 (Romil, GB). Solutions of particular metals were prepared using the analytical grade standard solutions: copper (II) nitrate trihydrate, lead (II) nitrate and zinc nitrate hexahydrate (Merck KGaA, Darmstadt, Germany) dissolved in deionized water. Verification of the obtained results was performed using two Certified Reference Materials (CRMs): NIST 1575a (Pine Needles) from the National Institute of Standards and Technology, Gaithersburg (plant), and NCS DC 73320 (soil) from the National Analysis Centre for Iron and Steel, Beijing, China. Both CRMs were analysed in every fifth measuring set.
Preparation of samples of Salix rhizosphere, roots and leaves for LMWOA analysis
Accordance to the method of Hammer and Keller (2002), the rhizosphere soil was sampled from the surroundings of the roots (soil attached to the roots after being shaken and separated from the roots by hand). The moist rhizosphere zone was separately preserved in polyethylene bags and transported to the laboratory. Subsamples of the field rhizosphere were mixed and dried at room temperature, and small pieces of broken roots and any other extraneous materials were carefully removed, prior to sieving <1 mm using a nylon fibre sieve, and stored for subsequent analysis, where 20 g of the samples were collected for extraction. The LMWOAs were extracted with 100 mL of water (pH = 2 acidified with concentrated HCl) in an orbital shaker at room temperature for 12 h. Organic acids from the water solution were extracted according to the modified method recommended by Baziramakenga et al. (1995). Extracts were filtered through Whatman No. 42 filters, and organic acids were extracted from the water solution three times with ethyl acetate (20 mL, 5 min). The volume of the solvent was reduced to 5 mL using a rotary evaporator at 40 °C after which it was transferred to an amber glass vial. The residue was rinsed from the flask with 1 mL of distilled water and added to the vial. The solvent was evaporated at room temperature under a stream of nitrogen to obtain 1 mL of aqueous solution.
After harvest, roots were immersed in 0.01 M HCl cold solution in order to eliminate trace elements adsorbed on the root surface (Adeniji et al. 2010). Subsequently, the roots were washed three times with cold distilled water and then gently dried on a filter paper to remove excess water. Plant organs (roots and leaves) were then prepared using the modified method of Adeniji et al. (2010) and Sanità di Toppi et al. (2007). The roots and leaves were severed from the shoot and fresh organs (approximately 1.0 g), ground to powder in a mortar chilled using liquid nitrogen, collected in 50-mL centrifuge tubes and stored frozen (−80 °C) until analysis. For analysis, 5 mL of H2O was added to the samples and the mixture was heated for 60 min in a water bath at 80 °C to denature the degradative enzymes. The mixture was then centrifuged at 3600 rpm/min for 15 min at 25 °C. Samples prepared from the rhizosphere, roots and leaves in 1 mL volume were transferred to the vial. For LMWOA determination, 10 μL of liquor was injected onto column C18.
Statistical analysis
The experimental data were analysed statistically using STATISTICA 12 software (StatSoft Inc.). In particular, an analysis of the expected value and standard deviation for metals and LMWOAs in two areas was performed. Additionally, one-factor analyses of variance for the efficiency of metal phytoextraction and acid accumulation in the rhizosphere, roots and leaves observed in Salix were made for the two different areas. The appropriate F statistics for tests of significance factor effects and for the interaction effect were applied. For significant differences the RIR Tukey test was carried out. For the studied areas, a one-way ANOVA analysis was performed, wherein the inter-group taxon was used as the factor, and the level of significance was λ = 0.05. In tables, the placed letters are the average content of elements, for which taxa display a significant difference in the average content of individual metals or LMWOAs, respectively in the rhizosphere, root and leaf. Analysis was performed separately for each of the studied areas. To compare the significance between metal contents in roots and leaves of tested Salix taxa growing at the two experimental areas (Area 1 and Area 2), Student’s t test was used.
Results
The analysis was performed to determine the difference between the contents of metals and LMWOAs in the rhizosphere, root and leaf for each studied area. It revealed significant differences between the contents of individual LMWOAs, metals and Salix taxa.
Characteristics of Cu, Pb and Zn contents in Salix organs
The results presented in Table 1 show a significant difference in the phytoextraction efficiency of the tested Salix taxa for Cu, Pb and Zn, growing in Area 1 and Area 2.
The highest content of Cu was observed in the leaves of S. × smithiana (S 207/191) collected from Area 1 (53.13 ± 3.67 mg kg−1 DW), whereas S. alba (S 225) and S. × purpurea 10 (S 58) from Area 2 (183.66 ± 12.51 and 172.26 ± 12.66 mg kg−1 DW, respectively) were Salix taxa with the highest content of Cu in this organ. S. × smithiana (S 207/191) growing in Area 1 was found to contain the highest amount of Pb in its leaves (11.54 ± 1.01 mg kg−1 DW), while in Area 2 the most effective plant to accumulate Pb (16.22 ± 0.74 mg kg−1 DW) was S. alba (S 225) (18.68 ± 0.68 mg kg−1 DW). The highest content of Zn was observed in the leaves of S. × smithiana (S 207/191) from Area 1, while in Area 2 the highest content of this metal was determined in S. purpurea 10 (S 58) leaves (199.96 ± 13.72 mg kg−1 DW).
Generally, the highest contents of Cu, Pb and Zn were detected in S. × smithiana (S 207/191) roots (116 ± 8.76, 87.84 ± 7.30 and 203.42 ± 14.62 mg kg−1 DW, respectively) collected from Area 2, whereas the highest contents of Pb and Zn (47.69 ± 3.52 and 60.95 ± 1.76 mg kg−1 DW, respectively) and a similar content of Cu (36.65 ± 2.35 mg kg−1 DW) were observed in S. viminalis ‘PR’ (S 305/305) collected from Area 1. It is worth underlining that S. alba (S 225) growing in Area 2 was found to be as highly efficient in Cu and Pb phytoextraction (105.46 ± 9.23 and 89.92 ± 9.96 mg kg−1 DW, respectively) as S. × smithiana (S 207/191) in its roots.
Concentration of organic acids in soil and plant samples
Among the ten analysed LMWOAs, oxalic, malic, acetic, citric and succinic acids were particularly detected in the analysed rhizosphere of the studied willow taxa (Table 2). Other acids were characterized by much lower concentration, with values below the limit of detection (LOD). The obtained results were strictly dependent on the studied Salix taxa and the area of plant growth. The total concentrations of LMWOAs in the rhizosphere were higher in Area 2, where metal pollution was much more pronounced (especially in the case of Cu) than Area 1. For plants growing in Area 1, the total LMWOA concentration in the rhizosphere was 2.29 ± 0.06 and 20.82 ± 4.25 μM 100 g−1 DW for female specimens of S. × smithiana (S 1) and S. viminalis ‘PR’ (S 305/305) taxa, while in Area 2 the content was 15.28 ± 1.40 and 73.48 ± 6.77 M 100 g−1 DW for S. purpurea × triandra × viminalis 2 (S 46) and male specimens of S. × smithiana (S 207/191) taxa, respectively. In this area, a significant increase was especially observed for oxalic, malic, acetic and citric acid (for many taxa almost/more than tenfold).
In the case of the profile and content of LMWOAs in Salix roots and leaves, the results also showed that the values were dependent on both factors: willow taxa and plant growth condition were strongly related (Tables 3, 4). In roots of Salix collected from Area 1, the total content of LMWOAs was 1.67 ± 0.07 and 12.95 ± 1.65 μM 100 g−1 DW for S. × rubra 4 (S 30) and S. viminalis ‘PR’ (S305/305) taxa whereas in roots of the plants from Area 2, the total content was 7.62 ± 0.81 and 49.79 ± 2.65 μM 100 g−1 DW for S. purpurea × triandra × viminalis 2 (S 46) and S. × rubra 4 (S 30) taxa, respectively. The oxalic, malic, acetic and citric acid contents increased in the roots of willows from Area 2 in comparison to willows from Area 1 (Table 3). In leaves, the total content of LMWOAs in plants from Area 1 was 3.46 ± 0.33 and 30.44 ± 2.63 μM 100 g−1 DW for S. × rubra 4 (S 30) and S. × smithiana (S 207/191) taxa, while in plants from Area 2 their content was 21.68 ± 4.12 and 78.12 ± 3.95 μM 100 g−1 DW for S. cinerea (S 205/189) and S. alba (S 225) taxa, respectively.
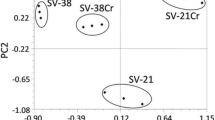
In addition, a cluster analysis using Ward’s method was made. As in one-way ANOVA, Ward’s method is used to estimate the distance between the clusters. Cluster analysis was performed for the content of metals and LMWOAs for the studied Salix taxon in Areas 1 and 2, respectively, in the rhizosphere, leaf and root. Ward’s method was conducted using the Euclidean distance (in order to determine the measure of similarity between the contents of acids and metals in Salix). Particular emphasis was placed on Cu, whose concentration in soil was the highest in Area 2 among the investigated metals (Fig. 1a). In roots, the content of Cu was similar in relation to Pb content or much lower than for Zn for most tested taxa (Fig. 1b), which may offer some evidence of the limited transport of Cu to roots. In the case of Area 1 the content of metals in the roots was significantly lower (Fig. 1b). Statistical analysis showed that the content of metal in the roots depends only on the studied area. However, cluster analysis performed in order to compare the contents of Cu, Pb and Zn in willow leaves, revealed significant differences in metal content as a result of both factors: location of plants’ growth (clean and polluted area) and tested willow taxon (Fig. 1c).
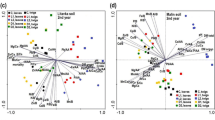
The clearest example is shown by the S. × smithiana (S 207/191) willow taxon, where the profile and concentration of LMWOAs in the rhizosphere and their content in the willow organs is strictly dependent on the studied area and taxon (Fig. 2). In Fig. 2a it can be seen that the maleic, citric and fumaric acids present in the rhizosphere from Area 1, and succinic acid present from both areas, were negligible. The first subgroup, of acids present at similar concentrations, contained oxalic and malic acid in Area 1, and formic, malonic, acetic and malic acids in Area 2. The second subgroup included formic, malonic, acetic and lactic acids present in Area 1, and the third subgroup, in which maleic, citric, fumaric and succinic acids were present in Area 2, are marked at a slightly higher concentration. It is interesting to note that oxalic acid, the concentration of which in the rhizosphere is small for Area 1, and was significantly increased in Area 2, created another fourth subgroup (Fig. 2b). It can be assumed that oxalic acid could exhibit a defence mechanism against Cu toxicity for the S. × smithiana (S 207/191) willow taxon. In turn, the analysis of LMWOAs content performed in roots divides acids into two main groups that are further split into two smaller subgroups. Within the first group differentiation between acids was significantly higher compared to the second group. Moreover, acids that played no significant role in the rhizosphere began to play crucial role in the root. Similar results have been statistically proven for S. purpurea × triandra × viminalis 2 (S 46), S. cinerea (S 205/189) and S. viminalis ‘PR’ (S 305/305) willow taxa. But for S. × smithiana (S 1), S. × rubra 4 (S 30), S. × smithiana 2 (S 44), S. × purpurea 10 (S 58) or S. alba (S 225) willow taxa, statistically important acids present in the rhizosphere, were also statistically important in the root. In leaves similar scattering results were observed to those observed for LMWOAs present in the roots (Fig. 2c). However, it was observed that for plants from Area 1 acetic, maleic, malonic and succinic acids began to assume an important role for leaves, and lactic, fumaric, acetic and formic acids for plants from Area 2. Results have also been statistically proven for S. purpurea × triandra × viminalis 2 (S 46), S. cinerea (S 205/189) and S. viminalis ‘PR’ (S 305/305) willow taxa. But for S. × smithiana (S 1), S. × rubra 4 (S 30), S. × smithiana 2 (S 44), S. × purpurea 10 (S 58) or S. alba (S 225) willow taxa, statistically important acids present in the rhizosphere, were also statistically important in the root.
Discussion
The concentrations of Cu and Zn in European arable soil layer are widely varied and strictly related to the local geochemical soil properties (Kabata-Pendias and Pendias 1999). However, contamination of soil with Cu and Zn is not such a serious problem as Pb or cadmium (Cd) contamination due to the differing toxicity of these metals; they may, however, constitute a significant local problem as a result of anthropogenic activities such as the extraction of mineral ores or the presence of heavy traffic.
The phytoextraction of elements in various Salix taxa has been discussed in numerous papers over recent years (Wieshammer et al. 2007; Kuzovkina and Volk 2009; Kersten 2015). Plants growing in unpolluted and polluted areas were characterized by higher or lower efficiency of element phytoextraction (Vysloužilová et al. 2003 Laidlaw et al. 2012). The results presented in this paper point to differences in metal translocation but there are limited data concerning the correlation between the phytoextraction of elements and the amount of LMWOAs exudated into the rhizosphere or the presence of these molecules in Salix organs. Moreover, the obtained results indicate significant differences in the phytoextraction of all three metals in plants growing in the two experimental areas, which could suggest a correlation between metals and acids (Magdziak et al. 2011). Additionally, significant differences between Salix taxa as regards their ability to extract the metals highlight the importance of plant species/varieties for the efficiency of this process (Mleczek et al. 2017).
The most promising plants for effective phytoextraction of Cu, Pb and Zn were S. alba (S 225) and male specimens of S. × smithiana (S 207/191). In the case of the first Salix taxon, its higher capacity for Cu and/or Pb and/or Zn phytoextraction has been discussed in some previous works (Borišev et al. 2009; Mleczek et al. 2010; Corneanu et al. 2014), which also described its limitations as regards the phytoextraction of some other elements, e.g. Cd (Ling et al. 2011). Additionally, S. alba was characterized in comparison with plants other than Salix, some of which were found to possess higher phytoextraction abilities. S. × smithiana has also been tested but the knowledge of its phytoextraction potential is generally limited (Iqbal et al. 2012; Puschenreiter et al. 2013). Kacálková et al. (2009) have shown the potential of this Salix taxon to extract Cu and Zn to be generally higher than Populus nigra × P. maximowiczii, and has a great potential in phytoremediation (Zalesny et al. 2006).
Mleczek et al. (2017) described the phytoextraction of all three metals in 145 Salix taxa growing in Grodziec Mały Village one year earlier. The authors characterized the efficiency of Cu, Pb and Zn phytoextraction in roots and leaves. When comparing the results, a general increase in the content of the analysed metals was observed in leaves with the higher diversity in metals content between analyzed plants. This suggests that the estimation of the real potential of particular Salix taxa for metal phytoextraction should be performed in the first 2–3 years of Salix growth, because the development of these plants is diverse. Generally, the contents of Cu, Pb and Zn in the tested soils were lower than the average concentrations of these metals in Polish soils (10.11 ± 19.94, 24.98 ± 74.95 and 79.81 ± 407.05 mg kg−1 DW, respectively) (Siebielec et al. 2012) as well as in the surface layer of European soils (17.3 and 68.1 mg kg−1 DW, respectively, for Cu and Zn) (Salminen et al. 2005). In spite of this, the efficiency of phytoextraction of all metals in Salix was particularly high in leaves. In many cases, the presented values were significantly higher than those recorded in other papers (Ahmed et al. 2015) and the transport of metals inside plants was more effective in relation to Salix taxa other than the ones studied in this paper, when comparing, e.g., translocation factor values (Yang et al. 2014).
The exudation of LMWOAs and their contents in Salix roots and leaves was strictly dependent on Salix taxon and the environmental conditions. Several LMWOAs, mainly oxalic, acetic and citric acids, have been shown to be the dominant root exudates at elevated levels of metals such as Al, Cd, Cu, Cr, Ni and Zn (Magdziak et al. 2011; Gąsecka et al. 2012; Drzewiecka et al. 2012; Goliński et al. 2015). In the present study, the Salix taxa growing in Area 2 were characterized by a high concentration of acids exudated into the rhizosphere, with the dominant acids in this case being oxalic, malic, acetic and citric. To our knowledge, this is the first time that LMWOAs have been studied in nine different Salix taxa growing on two different soils, and the data have been obtained as to which was the dominant acid(s) exudated by them. For this reason, higher levels of mainly oxalic and acetic acids could explain the response of willows growing in Area 2, as a specific mechanism to the physiological stress generated by the high-concentration metals in the soil. Environmental study also confirms our previous hydroponic study, which demonstrated that the presence of acetic and oxalic acids was due to elevated levels of Cu in modified Knop solution (Gąsecka et al. 2012). The increased concentration of acids may be explained as a defence mechanism consisting of metal detoxification by exudation of acids (Gąsecka et al. 2012). Moreover, all the studied Salix taxa were characterized by proper growth (without necrosis symptoms) and a high efficiency of Cu phytoextraction in their roots and leaves. Not without significance, LMWOAs are encountered as important root exudates, whose processes affect the rhizosphere. They are able to form complexes with metals and influence metal solubility, mobilization, and then uptake by plants (Parisová et al. 2013). The main determined acids in willow organs such as oxalic, malonic and citric are listed as molecules that are involved in the transport of metal through the xylem and vascular metal sequestration (Ueno et al. 2005; Parisová et al. 2013). Additionally, LMWOAs were detected in roots and leaves. This is due to their major role in several biochemical pathways, like photosynthesis, energy and respiration generation, cation transport, amino acid synthesis and metal detoxification (Schulze et al. 2002; Dresler et al. 2014). Rauser (1999) hypothesized that the content of LMWOAs in plant roots and/or shoots and their ability to tolerate metal are strictly correlated. However, this hypothesis is still being discussed (Wójcik 2009; Dresler et al. 2014; Goliński et al. 2015). The content of LMWOAs in plants mainly exposed to Cu or Cd has been presented by numerous authors (Chaffai et al. 2006; Dresler et al. 2014), whereas the influence of plant growth conditions on LMWOAs content in diverse Salix taxa roots and leaves has not been investigated. Our results clearly show that differences between two experimental areas and Salix taxa significantly influenced LMWOA content in plant organs. Doncheva et al. (2006) reported that the toxic effect of Cu was decreased by exogenous succinic acid in Zea mays L. growth, content of chlorophyll as well as by activities of antioxidant enzymes. The authors confirmed that succinic acid has a high affinity to Cu, which results in the reduction of Cu toxicity by the formation of a Cu-succinate complex. The appearance of succinic acid in Salix roots and leaves might, therefore, confirm their primary role in limiting the toxicity of Cu in Area 2.
Conclusions
The obtained results confirmed that the higher concentration of elements in soil from Area 2 was related with the higher concentration of LMWOAs in the rhizosphere, roots and leaves and finally with the higher content of metals in plant organs. Metals present in soil from Area 2 induced a significant increase (p > 0.05) in the concentrations of oxalic, malic and acetic acids in the rhizosphere, and in the contents of oxalic, malic, acetic and citric acids in roots and leaves. It may be safely assumed that detoxification of metals (Cu, Pb and Zn) in the studied taxa involves both the exudation of LMWOAs from roots to the rhizosphere and internal detoxification, probably by the formation of complex metal—LMWOAs. Data on the higher content of LMWOAs in roots and leaves under higher metal concentration in soil from Area 2 suggest that organic acids play an important role in contributing to the metal chelation process and their sequestration into the studied organs.
Author contribution statement
ZM is the corresponding author and participated in the analysis of low-molecular weight organic acids, statistical analysis, manuscript preparation and experiment preparation. MM participated in manuscript and experiment preparation. PR participated in plant material and manuscript preparation. PG participated in manuscript preparation.
References
Adeniji BA, Budimir-Hussey MT, Macfie SM (2010) Production of organic acids and adsorption of Cd on roots of durum wheat (Triticum turgidum L. var. Durum). Acta Physiol Plant 32:1063–1072. doi:10.1007/s11738-010-0498-6
Ahmed W, Ahmad M, Rauf A, Shah F, Khan S, Kamal S, Shah S, Khan A (2015) Evaluations of some trace metal levels from the leaves of Salix nigra in Hayatabad Industrial Estate Peshawar, Khyber Pakhtunkhwa Pakistan. Am J Biomed Life Sci 3:21–24. doi:10.11648/j.ajbls.s.2015030201.13
Albarracín VH, Amoroso MJ, Abate CM (2010) Bioaugmentation of copper polluted soil microcosms with Amycolatopsis tucumanensis to diminish phytoavailable copper for Zea mays plants. Chemosphere 79:131–137. doi:10.1016/j.chemosphere.2010.01.038
Baziramakenga R, Simard RR, Leroux GD (1995) Determination of organic acids in soil extracts by ion chromatography. Soil Biol Biochem 27:349–356. doi:10.1016/0038-0717(94)00178-4
Borišev M, Pajević S, Nikolić N, Pilipović A, Krstić B, Orlović S (2009) Phytoextraction of Cd, Ni, and Pb Using Four Willow Clones (Salix spp.). Pol J Environ Stud 18:553–561
Chaffai R, Tekitek A, El Ferjani E (2006) A comparative study on the organic acid content and exudation in maize (Zea mays L.) seedlings under conditions of copper and cadmium stress. Asian J Plant Sci 5:598–606. doi:10.3923/ajps.2006.598.606
Chen YX, Lin Q, Luo YM, He YF, Zhen SJ, Yu YL, Tian GM, Wong MH (2003) The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 50:807–811. doi:10.1016/S0045-6535(02)00223-0
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486. doi:10.1007/s004250000458
Corneanu M, Hernea C, Butnariu M, Corneanu G, Sărac I, Hollerbach W, Neţoiu C, Petcov AA (2014) Preliminary tests for Salix sp. tolerance to heavy metals (Cd, Ni, Pb). Geophysical Research Abstracts 16, EGU2014-10620, EGU General Assembly
Dakora F, Phillips D (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47. doi:10.1023/A:1020809400075
Doncheva S, Stoyanova Z, Georgieva K, Nedeva D, Dikova R, Zehirov G, Nikolova A (2006) Exogenous succinate increases resistance of maize plants to copper stress. J Plant Nutr Soil Sci 169:247–254. doi:10.1002/jpln.200520560
Dresler S, Bednarek W, Wójcik M (2014) Effect of cadmium on selected physiological and morphological parameters in metallicolous and non-metallicolous populations of Echium vulgare L. Ecotox Environ Safe 104:332–338. doi:10.1016/j.ecoenv.2014.03.019
Drzewiecka K, Mleczek M, Gąsecka M, Magdziak Z, Goliński P (2012) Changes in Salix viminalis L. cv. ‘Cannabina’ morphology and physiology in response to nickel ions—Hydroponic investigations. J Hazard Mater 217–218:429–438. doi:10.1016/j.jhazmat.2012.03.056
Duarte B, Freitas J, Caçador I (2011) The role of organic acids in assisted phytoremediation processes of salt marsh sediments. Hydrobiologia 674:169–177. doi:10.1007/s10750-011-0731-3
Gąsecka M, Mleczek M, Drzewiecka K, Magdziak Z, Rissmann I, Chadzinikolau T, Golinski P (2012) Physiological and morphological changes in Salix viminalis L. as a result of plant exposure to copper. J Environ Sci Heal A 74:33–40. doi:10.1080/10934529.2012.650557
Ghnaya T, Zaier H, Baioui R, Sghaier S, Lucchini G, Sacchi GA, Lutts S, Abdelly Ch (2013) Implication of organic acids in the long-distance transport and the accumulation of lead in Sesuvium portulacastrum and Brassica juncea. Chemosphere 90:1449–1454. doi:10.1016/j.chemosphere.2012.08.061
Goliński P, Mleczek M, Magdziak Z, Gąsecka M, Borowiak K, Dąbrowski J, Kaczmarek Z, Rutkowski P (2015) Efficiency of Zn phytoextraction, biomass yield and formation of low-molecular-weight organic acids in S × rubens—a hydroponic experiment. Chem Ecol 31:345–364. doi:10.1080/02757540.2014.993976
Guidi Nissim W, Labrecque M (2016) Planting microcuttings: an innovative method for establishing a willow vegetation cover. Ecol Eng 91:472–476. doi:10.1016/j.ecoleng.2016.03.008
Hammer D, Keller C (2002) Changes in the rhizosphere of metal-accumulating plants evidenced by chemical extractants. J Environ Qual 31:1561–1569. doi:10.2134/jeq2002.1561
Iqbal M, Puschenreiter M, Oburger E, Santner J, Wenzel WW (2012) Sulfur-aided phytoextraction of Cd and Zn by Salix smithiana combined with in situ metal immobilization by gravel sludge and red mud. Environ Pollut 170:222–231. doi:10.1016/j.envpol.2012.07.008
Janicka-Russak M, Kabala K, Burzynski M, Klobus G (2008) Response of plasma membrane H+-ATPase to heavy metal stress in Cucumis sativus root. J Exp Bot 59:3721–3728. doi:10.1093/jxb/ern219
Jones D, Darrah P (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257. doi:10.1007/BF00008338
Kabata-Pendias A, Pendias H (1999) Biogeochemia pierwiastków śladowych. Wydawnictwo Naukowe PWN, Warszawa
Kacálková L, Tlustoš P, Száková J (2009) Phytoextraction of cadmium, copper, zinc and mercury by selected plants. Plant Soil Environ 55:295–304
Kersten G (2015) Phytoremediation of Metal Contamination using Salix (willows). Electronic Theses and Dissertations. Paper 1034. University of Denver, Digital Commons @ DU
Kutrowska A, Szelag M (2014) Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals. Acta Physiol Plant 36:1957–1968. doi:10.1007/s11738-014-1576-y
Kuzovkina YA, Volk TA (2009) The characterization of willow (Salix L.) varieties for use in ecological engineering applications: co-ordination of structure, function and autecology. Ecol Eng 35:1178–1189. doi:10.1016/j.ecoleng.2009.03.010
Laidlaw WS, Arndt SK, Huynh TT, Gregory D, Baker AJM (2012) Phytoextraction of heavy metals by willows growing in biosolids under field conditions. J Environ Qual 41:134–143. doi:10.2134/jeq2011.0241
Ling T, Jun R, Fangke Y (2011) Effect of cadmium supply levels to cadmium accumulation by Salix. Int J Environ Sci Tech 8:493–500. doi:10.1007/BF03326235
Liu J, Xiong Z, Li T, Huang H (2004) Bioaccumulation and ecophysiological responses to copper stress in two populations of Rumex dentatus L. from Cu contaminated and non-contaminated sites. Environ Exp Bot 52:43–51. doi:10.1016/j.envexpbot.2004.01.005
Magdziak Z, Kozlowska M, Kaczmarek Z, Mleczek M, Chadzinikolau T, Golinski P, Drzewiecka K (2011) Influence of Ca/Mg ratio on phytoextraction properties of Salix viminalis. II. Secretion of low molecular weight organic acids to the rhizosphere. Ecotox Environ Safe 74:33–40. doi:10.1016/j.ecoenv.2010.09.003
Martins N, Gonçalves Andrade PB, Valentão P, Romano A (2013) Changes on organic acid secretion and accumulation in Plantago almogravensis Franco and Plantago algarbiensis Samp. under aluminum stress. Plant Sci 198:1–6. doi:10.1016/j.plantsci.2012.09.001
Meier S, Alvear M, Borie F, Aguilera P, Ginocchio R, Cornejo P (2012) Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotox Environ Safe 75:8–15. doi:10.1016/j.ecoenv.2011.08.029
Mleczek M, Rutkowski P, Rissmann I, Kaczmarek Z, Goliński P, Szentner K, Stróżyńska K, Stachowiak A (2010) Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenerg 34:1410–1418. doi:10.1016/j.biombioe.2010.04.012
Mleczek M, Rutkowski P, Kaczmarek Z, Goliński P, Szentner K, Waliszewska B, Stolarski M, Szczukowski S (2017) Biological diversity of Salix taxa in Cu, Pb and Zn phytoextraction from soil. Int J Phytoremediat 19:121–132. doi:10.1080/15226514.2016.1207597
Mocek A, Drzymała S (2010) Geneza, analiza i klasyfikacja gleb (Genesis, analysis and soil classification). Wydawnictwo UP, Poznań (in Polish)
Neumann G, Rőmheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130. doi:10.1023/A:1004380832118
Nigam R, Srivastava S, Prakash S, Srivastava M (2001) Cadmium mobilization and plant availability—the impact of organic acids commonly exuded from roots. Plant Soil 230:107–113. doi:10.1023/A:1004865811529
Parisová M, Navrátil T, Šestáková I, Dytrtová JJ, Mareček V (2013) Influence of Low Molecular Weight Organic Acids on Transport of Cadmium and Copper Ions across Model Phospholipid Membranes. Int J Electrochem Sci 8:27–44
Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6:171–180. doi:10.1016/S1367-5931(02)00298-3
Puschenreiter M, Wittstock F, Friesl-Hanl W, Wenzel WW (2013) Predictability of the Zn and Cd phytoextraction efficiency of a Salix smithiana clone by DGT and conventional bioavailability assays. Plant Soil 369:531–541. doi:10.1007/s11104-013-1597-0
Rauser WE (1999) Structure and function of metal chelators produced by plants. Cell Biochem Biophys 31:19–48. doi:10.1007/BF02738153
Rutkowski P (ed) (2013) Willows (Salix) of Poznan University of Life Sciences collection. Wyd. Uniw. Przyr. w Poznaniu, Poznań, p 201
Salminen R et al (eds) (2005) Geochemical Atlas of Europe. Part 1—Background Information, Methodology and Maps. Geological Survey of Finland, Otamedia Oy, Espoo, p 525
Sanità di Toppi L, Vurro E, Rossi L, Marabottini R, Musetti R, Careri M, Maffini M, Mucchino C, Corradini C, Badiani M (2007) Different compensatory mechanisms in two metal-accumulating aquatic macrophytes exposed to acute cadmium stress in outdoor artificial lakes. Chemosphere 68:769–780. doi:10.1016/j.chemosphere.2006.12.092
Schulze J, Tesfaye M, Litjens RHMG, Bucciarelli B, Trepp G, Miller S, Samac D, Allan D, Vance CP (2002) Malate plays a central role in plant nutrition. Plant Soil 247:133–139. doi:10.1023/A:1021171417525
Senden MHMN, Wolterbeek HT (1990) Effect of citric acid on the transport of cadmium through xylem vessels of excised tomato stem-leaf systems. Acta Bot Neerl 39:297–303. doi:10.1111/j.1438-8677.1990.tb01399.x
Siebielec T, Smreczek B, Klimkowicz-Pawlas A, Maliszewska-Kordybach B, Terelak H, Koza P, Hryńczuk B, Łysiak M, Miturski T, Gałązka R, Suszek B (2012) Monitoring chemizmu gleb ornych w Polsce w latach 2010–2012. Państwowy Instytu Badawczy w Puławach, Poland, pp 94–101
Tiffin LO (1970) Translocation of iron citrate and phosphorus in xylem exudate of soybean. Plant Physiol 45:280–283
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotox Environ Safe 113:271–278. doi:10.1016/j.ecoenv.2014.12.014
Ueno D, Ma JF, Iwashita T, Zhao FJ, McGrath SP (2005) Identification of the form of Cd in the leaves of a superior Cd-accumulating ecotype of Thlaspi caerulescens using 113Cd-NMR. Planta 221:928–936. doi:10.1007/s00425-005-1491-y
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant, Cell Environ 13:195–206. doi:10.1111/j.1365-3040.1990.tb01304.x
Vivas A, Barea JM, Biró B, Azcón R (2006) Effectiveness of autochthonous bacterium and mycorrhizal fungus on Trifolium growth, symbiotic development and soil enzymatic activities in Zn contaminated soil. J Appl Microbiol 100:587–598. doi:10.1111/j.1365-2672.2005.02804.x
Vysloužilová M, Tlustoš P, Száková J (2003) Cadmium and zinc phytoextraction potential of seven clones of Salix spp. planted on heavy metal contaminated soils. Plant Soil Environ 49:542–547
Wei W, Wang Y, Wei ZG, Zhao HY, Li HX, Hu F (2009) Roles of organic acids and nitrate in the long-distance transport of cobalt in xylem saps of Alyssum murale and Trifolium subterraneum. Biol Trace Elem Res 131:165–176. doi:10.1007/s12011-009-8360-7
Wieshammer G, Unterbrunner R, García TB, Zivkovic MF, Puschenreiter M (2007) Phytoextraction of Cd and Zn from agricultural soils by Salix ssp. and intercropping of Salix caprea and Arabidopsis halleri. Plant Soil 298:255–264. doi:10.1007/s11104-007-9363-9
Wójcik M (2009) Vacuole as a multifunctional compartment in plant responses to stress factors. In: Maksymiec W (ed) Compartmentation of responses to stresses in higher plants, true or false. Transworld Research Network, Kerala, pp 91–123
Yang WD, Wang YY, Zhao FL, Ding ZL, Zhang XC, Zhu ZQ, Yang XE (2014) Variation in copper and zinc tolerance and accumulation in 12 willow clones: implications for phytoextraction. J Zhejiang Univ Sci B 15:788–800. doi:10.1631/jzus.B1400029
Zalesny RS Jr, Wiese AH, Bauer EO, Riemenschneider DE (2006) Sapflow of hybrid poplar (Populus nigra L. × P. maximowiczii A. Henry ‘NM6’) during phytoremediation of landfill leachate. Biomass Bioenerg 30:784–793. doi:10.1016/j.biombioe.2005.08
Zeng X, Li L, Mei X (2008) Heavy metal content in Chinese vegetable plantation land soils and related source analysis. Agric Sci China 7:1115–1126. doi:10.1016/S1671-2927(08)60154-6
Acknowledgements
The authors wish to acknowledge the financial support provided for this study by the National Centre for Research and Development under Project Grant No. R12 0065 10.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. G. dos Santos.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Magdziak, Z., Mleczek, M., Rutkowski, P. et al. Diversity of low-molecular weight organic acids synthesized by Salix growing in soils characterized by different Cu, Pb and Zn concentrations. Acta Physiol Plant 39, 137 (2017). https://doi.org/10.1007/s11738-017-2434-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2434-5