Abstract
Submersed Callitriche cophocarpa is an outstanding Cr phytoremediator in water systems. The mineral elements in waters can penetrate the submersed plant surface. This has led us to the hypothesis that the absorbed Cr can alter the mechanical properties of leaves. These properties were measured by applying atomic force microscopy. C. cophocarpa shoots were immersed in 100 µM (5.2 mg/l) Cr solution for 7 days. Cr was applied independently at two distinct oxidation states as Cr(VI) and Cr(III), known from different physicochemical properties and toxic effects. The contents of elements which were proportional to the fluorescence signal in individual leaves were evaluated using micro-X-ray fluorescence spectroscopy. The results obtained showed that the leaf epidermis significantly changes its elastic properties upon incubation with Cr-supplemented solution. When compared to the control, a drop in the leaf’s stiffness observed for Cr(III) was ca. 42 %. In the case of Cr(VI)-treated leaves, the stiffness raised to ca. 17 %. The changes in elasticity were significantly correlated with the contents of Ca (Pearson’s coefficient r = 0.87, p < 0.017). The results led us to ascertain that it is Cr(III) but not Cr(VI) that significantly influences Ca removal from leaves thus decreasing the stiffness of the leaf’s epidermis.
Similar content being viewed by others
Introduction
The chromium content in surface water is usually at a low level, typically between 0.3 and 6 μg/l. Wastewater originating from steel manufacturing, chrome plating and tanneries, however, can be highly polluted by Cr compounds, up to the level of a few hundred mg/l (Sperling et al. 1992; Kyzioł-Komosińska et al. 2008). These concentrations reflect a total chromium content comprising a rich variety of chemical species. The availability of chromium ions to organisms as well as their toxic effects depends strongly on chromium oxidation states. There are two stable Cr species in the environment: Cr(III) and Cr(VI). Cr(III) is considered to be a micronutrient, essential for the maintenance of the glucose and lipid metabolism in mammals (Schwartz and Mertz 1959). Although when it exceeds the permitted concentration in water, i.e., 50 μg/l (Regulation of 9 November, 2011), it may negatively influence aquatic life and, after consumption, leads to human health problems (Codd et al. 2001). Cr(VI) is highly toxic to biota owing to its oxidizing potential and easy permeation of biological membranes (Kimbrough et al. 1999; Codd et al. 2001; Dhal et al. 2013). Cr(VI) and Cr(III) can interconvert (Kotaś and Stasicka 2000), and the reduction of Cr(VI) to Cr(III) is assumed to be the main detoxification mechanism of Cr(VI) (Zayed and Terry 2003). Both forms of Cr are regarded by the Environmental Protection Agency (USA) as priority toxic pollutants.
The Callitriche cophocarpa, a widely distributed aquatic higher plant (macrophyte), was recently proven to be an excellent candidate for both the Cr(III) and Cr(VI) phytoextraction purpose (Augustynowicz et al. 2010, 2013). This submersed macrophyte is of great interest as regards the phytoremediation of polluted water for two key reasons. Firstly, it is able to accumulate pollutants in its shoots, and thus, the pollution could be effectively removed with plant biomass without any loss in the root-sediment system. It must be stressed that little information is available on the topic of chromium remediation by shoots of aquatic vegetation. Secondly, it is known that submerged macrophytes can reveal a higher metal uptake than floating aquatic plants due to a high contact area with the surrounding water (Chandra and Kulshreshtha 2004). C. cophocarpa reveals the extraordinary accumulation capacity of Cr ions comparable with those described for commercially used sorbents (Augustynowicz et al. 2013).
Submersed plant species can uptake mineral elements from water reservoirs and sediment. This process can undergo through the surface of leaves, which leads us to the hypothesis that Cr uptake can alter the mechanical properties of leaves. In the present study, in order to verify this hypothesis, we have used atomic force microscopy (AFM) (Vileno et al. 2007) to determine the mechanical properties of C. cophocarpa leaves exposed to 100 µM concentration of Cr(III) and Cr(VI). We have shown an important correlation between Cr(VI), Cr(III) and Ca content in relation to the stiffness of the leaf. In order to determine content of Cr and Ca elements in an individual leaf, we used micro-X-ray fluorescence spectroscopy (µXRF). This method enables the measurement of the intensity of the fluorescence signal of the particular element, which is proportional to the element content. In this study, we attempted to find a new basic mechanism of Cr influence on Callitriche leaves, but we did not focus on the applicability of the results in the context of the phytoremediation technology, which was rather the subject of our earlier works (Augustynowicz et al. 2010, 2013, 2014). We believe that this study will contribute to the understanding of the mechanisms controlling the influence of heavy metal ions on higher plant species in the aquatic environment.
Materials and methods
Plant material and incubation in Cr media
Callitriche cophocarpa Sendtn. (water starwort) was collected from the Dłubnia river, southern Poland: 50º16′N/19º56′E, during the vegetation season of the year 2012. Mature shoots of about 10 cm in length were rinsed several times with distilled water after tap water and used for further experiments. The incubation of plants in Cr media was performed over a period of 7 days. Cr solutions were prepared using water derived from the natural stands of plants, supplemented with 100 µM (5.2 mg/l) Cr(VI) (as K2Cr2O7) or Cr(III) (as Cr2(SO4)3·18 H2O) (POCh Gliwice, Poland). Before the experiment, the water was filtered to prevent the growth of microorganisms (Supelco filters, 0.2 μm pore size). The Cr concentration was selected according to earlier experiments (Augustynowicz et al. 2010, 2013). Plants exposed to this Cr concentration exhibited only some symptoms of physiological stress. ICP-MS spectroscopy (ELAN 6100, Perkin Elmer) (PN-EN ISO 9963-1:2001) calibrated against the ICP multi-element standard (Merck) and titration methods (PN-ISO 9297:1994, PN-EN ISO 17294-1:2007) was applied to measure the chemical composition of the water. The average amount (mg/l) of elements were as follows: 4.24 Na+, 1.75 K+, 69.65 Ca2+, 5.01 Mg2+, 2 × 10−3 Fe2+, 5 × 10−3 Mn2+, 5 × 10−3 Zn2+, 6 × 10−4 Cu2+, 10−3 Mo6+, 16.50 Cl−, 10.20 SO4 2−, 189.00 HCO3 2−, 13.50 NO3 2−, 0.15 PO4 3− and 0.08 BO3 3−. The level of heavy metals, Pb, Hg, Cd, Zn, Tl, Ni and Cr, did not exceed 1 μg/l. The average electrical conductivity of water was equal to 0.335 mS/cm, pH = 7.8 and Eh (redox potential) = 180 mV. An amount of 1.5 g of shoots was submersed in 300 ml of the aforementioned Cr solutions. Plants were incubated in the fitotron for 16 h with a light intensity of 35 μmol m−2 s−1 (LF 36 W/54, Piła, Poland) and 8 h of darkness at 23 °C. Plants were collected from the natural stand, which was a river quite strongly shaded by rich coastal vegetation (trees and shrubs). Therefore, at the time of sample collection (May–July), the submersed plants were not exposed to high light intensity. The photon flux used in these experiments was comparable with that reaching the shoots at the time when the plants were collected. Control samples were those plants incubated as described above but with no Cr addition. The analyses were carried out on mature leaves.
Atomic force microscopy (AFM)
The elastic properties of leaves were measured using a commercially available device (PSIA XE120, Park Systems, Korea) equipped with the “liquid cell” setup (Vileno et al. 2007). Standard silicon cantilevers in the conical shape of a probing tip were used in all experiments (NSC36, Park Systems). These were characterized by the 10-nm diameter of the tip radius and the cantilever spring constant of 1.75 N/m. In the AFM, a probing tip, mounted at the end of a delicate cantilever, indents the cell which results in a certain deflection of the cantilever. The deflection is measured using the laser–photodiode system (Fig. 1a). A photodiode, whose active area is divided into four quadrants, records the intensity of a laser beam deflected from the end of the cantilever (Fig. 1b). The magnitude of the deflection depends on the elastic properties of the sample. The leaf’s stiffness was determined by fitting a line to the part of the force–displacement curve, i.e., to the fragment between 0 and 100 nm, denoting the leaf’s 100-nm layer of thickness (Fig. 1c). The average values of the leaf’s stiffness were calculated from the fit of the Gauss function. In such case, the center of distribution denotes the mean value while half of its full width taken at half maximum (FWHM) is a standard deviation.
a The top view image of an AFM probing cantilever placed over a leaf’s surface. The elastic properties were collected from randomly selected regions by setting grids with the size of 25 × 25 µm2. b The scheme of the experimental setup. c Force–displacement curve reflecting the elastic properties of a studied leaf. The leaf’s stiffness is a slope obtained from linear regression and expressed in N/m
During AFM measurements, two sides of mature leaves were measured: the adaxial (upper surface) and abaxial (lower surface). Each time, ten different locations, in the middle part of the leaf on both sides of the main vascular bundle, with a scan size of 25 × 25 µm2, were probed in three various leaves. This experiment was repeated in three independent series. During AFM measurements, leaves were dipped into incubation solution.
Micro-X-ray fluorescence spectroscopy (µXRF)
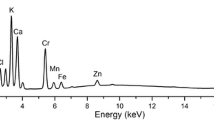
The abundance of chromium and calcium was studied using a laboratory µXRF setup consisting of a low-power X-ray tube with a molybdenum anode and silicon drift detector (SDD detector) described in detail elsewhere (Wróbel et al. 2012). The image of the experimental setup is presented in Fig. 2.
The angle between the impinging beam and the sample normal was 45°. Similarly, the angle between the detector axis and the sample normal was 45°. The X-ray tube voltage and current were 50 kV and 1 mA, respectively. Primary radiation from the X-ray tube was focused using a polycapillary lens into a Gaussian-shaped beam (Węgrzynek et al. 2008). The size of the focal spot was 16.4 μm taken at FWHM (full width of half maximum), and the size of the irradiated area was 380 μm2. The time of the measurements was 1–1.5 s per point. The samples were mounted between two Mylar films (with a thickness of 2.5 μm) stretched on the plastic holder. The holder was mounted on the motorized stage which allowed the three-directional movement of the sample with micrometer resolution. The typical size of the map was 1–1.5 mm2, and step sizes—the same in both the horizontal and vertical direction—were equal to 20 μm. The average time of imaging of a single sample was 4.5 h. Before analysis, the leaves were exhaustively washed in distilled water, gently dried and immediately dipped into liquid nitrogen. The samples were then freeze-dried (Alpha 1–4 Martin Christ Gefriertrocknungsan-lagen GmbH lyophyliser, Germany) to avoid the dehydration and redistribution of elements as a result of extended measurements. The intensity of X-ray emission is proportional to the element content. Thus, the intensity of X-ray emission (measured as counts per second; cps) at the given energy typical for the studied element, recorded by SDD detector from the irradiated area (cps/µm2), was used to determine the content of the elements in C. cophocarpa leaves. Up to eight independent leaves were analyzed, in three independent series, with n ∈ < 5,600, 40,000 > independently counted points.
We used the same plants, and we measured the same leaves ontogenetically and similar leaf parts in both AFM and µXRF analyses.
Statistics
One-way ANOVA or Kruskal–Wallis nonparametric ANOVA (if data are not normally distributed) and Tukey’s or Dunn’s tests, respectively, were performed to determine the statistical significance of the results. The correlation coefficient (r) was calculated according to Pearson’s correlation. All procedures were carried out at the significance level of α = 0.05, based on STATISTICA version 10 software (StatSoft Inc. 2011).
Results
The elastic properties of the leaf epidermis
The AFM measurements of the leaf epidermis showed significant differences in the mechanical properties of leaves exposed to chromium solutions as compared to untreated leaves. The statistical analysis (Kruskal–Wallis nonparametric ANOVA and Dunn’s test) revealed significant differences between the tested objects: control, Cr(III)- and Cr(VI)-treated leaves (in all cases p < 0.001). However, no statistical differences were observed between the elastic properties of upper (adaxial side) and lower (abaxial side) leaf epidermis in Cr-treated leaves (Table 1).
The average stiffness of untreated C. cophocarpa leaves was 0.98 ± 0.16 N/m (Fig. 3a). When the cantilever is pushed against a stiff, non-deformable surface, the slope reflects the stiffness of the cantilever used. Therefore, the maximum value of the stiffness measured by AFM depends on the spring constant of the cantilever. In our case, this was 1.75 N/m, which is almost two times higher than the stiffness of untreated leaves. Upon incubation with Cr-supplemented solutions, the measured mean elasticity increased (Fig. 3b) or decreased significantly (Fig. 3c). This indicated the softening or stiffening of the leaf’s surface, respectively. When compared to the control, the average drop of the leaf stiffness observed for Cr(III) was about 42 % (Fig. 3b). In the case of Cr(VI)-treated leaves, the average stiffness increased to ca. 17 % (Fig. 3c).
Elements analysis by µXRF
The fluorescence signal reflecting the amount of Cr considerably depended on the element oxidation state. The determined X-ray intensities of the Cr signal of Ca and Cr in leaves following exposure to Cr(III) and Cr(VI) were presented in Fig. 4. The intensity of the fluorescence signal of the particular element was proportional to the element content.
The average X-ray intensity (cps/µm2) reflecting contents of Cr and Ca in the control leaves and following the incubation of plants in Cr(III) and Cr(VI) solutions (represented as a mean and standard deviation). Different letters indicate significant statistical differences for Cr and Ca separately according to the ANOVA and Tukey’s tests (α = 0.05 for both tests). Values with the same letter are not significantly different
The average X-ray intensity for Cr detected in Cr(III)-treated leaves (0.404 ± 0.097 cps/µm2) was around 25 times higher as compared to the samples treated with Cr(VI) (0.016 ± 0.004 cps/µm2). The intensity of X-ray fluorescence for control leaves was at the level of 0.003 cps/µm2, and it was negligible as compared to Cr-treated samples. Furthermore, the µXRF analysis revealed the speciation-dependent influence of chromium on calcium levels. The X-ray fluorescence intensity for Ca in control leaves was at the level of 0.332 ± 0.135 cps/µm2. The intensity of X-ray emission for Ca decreased significantly following the exposure of leaves to Cr(III). When compared to the control, the drop was about 32 % (from 0.332 ± 0.135 to 0.239 ± 0.027 cps/µm2). On the other hand, when the leaves were exposed to Cr(VI), the fluorescence of the Ca signal increased up to 40 % (from 0.332 ± 0.135 to 0.437 ± 0.162 cps/µm2). The statistical difference between the studied Cr and Ca fluorescence signals in Cr-treated leaves was verified using ANOVA and Tukey’s tests. ANOVA was applied to compare variance, while Tukey’s test was used for post hoc means comparison. The ANOVA test showed the significant statistical difference at a level of α = 0.05 (Cr: p < 0.01, Ca: p < 0.01). Tukey’s test (α = 0.05) confirmed the statistical significance between the studied groups of leaves. The p value for Cr content in the studied groups was 0.000022 for Cr(III)-treated versus Cr(VI)-treated leaves and for Cr(III)-treated versus control groups. For the Cr(VI)-treated leaves compared to control leaves, p < 0.013781. The statistical analysis of the Ca content in all the groups of leaves studied delivers p < 0.000022.
Correlation between element content and the leaf’s stiffness
The correlation between the Ca fluorescence signal reflecting the element content and the leaf’s stiffness was confirmed by Pearson’s correlation coefficient, which is a measure of the linear correlation (dependence) between two variables. In this case, the Pearson coefficient was equal to 0.87 (p < 0.017). It means the higher the calcium concentration, the higher the leaf’s rigidity (Fig. 5).
Discussion
The aim of this study was the examination of the physical properties of the plant tissue exposed to an elevated concentration of Cr. Callitriche cophocarpa, which was chosen as an excellent model for this investigation. We would like to stress that the C. cophocarpa is an outstanding Cr accumulator. On the condition used in this experiment, this plant species can accumulate ca. 1,000 mg/kg d.w. after incubation in Cr(VI) (Augustynowicz et al. 2010). Moreover, the accumulation levels following exposure to Cr(III) are usually 5–10 times higher, and, up to now, the maximum detected level of Cr was almost 0.3 % d.w. (Augustynowicz et al. 2013). In accordance with the definition of hyperaccumulation (Van der Ent et al. 2013), when it grows in the natural environment, the C. cophocarpa would be called a Cr hyperaccumulator. Lastly, we also showed that C. cophocarpa is also able to phytoextract extremely toxic thallium and cadmium compounds, what may also find further biotechnological application (Augustynowicz et al. 2014). The objective of this study, however, was the investigation of the basic mechanism engaged in Cr influence on the plant structure, which has a direct influence on its metabolism in polluted water.
This submersed aquatic plant species is able to accumulate heavy metal compounds using the entire surface of its shoots. This means that the leaf’s epidermis on both the adaxial and abaxial sides, which were analyzed by AFM, were the first cell layers exposed to the heavy metal compounds. We commenced our study with the measurement of the leaf’s elastic properties upon the incubation of plants in the two most stable Cr oxidation forms in the environment. It should be stressed that the conditions of this study were environmentally relevant: Cr concentration in the solution was carefully chosen to induce some stress symptoms but without significant negative influence on the plant’s physiology during the time of experiment (Augustynowicz et al. 2010, 2013). Both methods, the AFM and µXRF, were consistent with respect to the tested object, i.e., the analyses were performed with the use of precisely selected mature plant leaves (please see Materials and Methods section). Callitriche leaves (ca. 2–4 mm wide and 10 mm long) are very delicate and well hydrated (water constitutes 93 % of the leaf biomass). Therefore, during the AFM measurements, the “liquid cell” setup was used, which enabled the conduction of experiments on the leaf samples submersed in the incubation medium. In turn, µXRF is the technique that allows the analysis of element concentration at a very low level (Punshon et al. 2009), typical for the single leaf. In order to determine the amount of elements in tissue, we measured the X-ray intensity (cps/µm2) of the Cr and Ca signal since the intensity of the fluorescence signal of the particular element is proportional to the element content.
The AFM measurements of the leaf epidermis showed significant differences in the mechanical properties of leaves exposed to chromium solutions as compared to untreated leaves. Cr(III)-treated leaves exhibited the lowest stiffness indicating softening upon Cr(III) exposure. However, leaves treated with Cr(VI) showed a substantial increase in rigidity, which denoted the hardening of a leaf’s surface as compared to non-treated leaves. These results correlated well with the determination of Cr content, which was the largest for Cr(III)-treated leaves. We attempted to explain the observed changes in the elasticity of the leaves caused by different Cr species, and we found that calcium content is significantly influenced in plant material exposed to Cr. Calcium (Ca2+) ions are a key element that plays a role in various processes, such as maintaining osmotic pressure within vacuoles, the regulation of enzyme activity and participation in signaling cascades as secondary messengers. Moreover, Ca is known to be a very important structural element stabilizing cell membranes and strengthening cell walls due to its bounding to negatively charged functional groups (Ubarretxena-Belandia et al. 1998; White and Broadley 2003). In the control, i.e., intact leaves, high Ca and low Cr levels were observed. Upon exposure to Cr, the plant accumulated Cr from the solution. However, Cr uptake strongly influenced the quantity of Ca in the leaves. The accumulation of Cr in Cr(III)-exposed leaves was accompanied by the significant Ca reduction reflected by the decrease of the Ca fluorescence signal. Contrary to this result, the incubation of plants in Cr(VI) caused an increase in Ca content as compared to the control. The observed phenomenon could be explained on the basis of the physiochemical properties of the investigated elements, which are discussed below.
In an aquatic environment, Cr(III) occurs as a cation, usually in the form of hydroxyl complexes (e.g., CrOH2+). Its transport through the cell membrane does not require the use of metabolic energy (Zayed and Terry 2003). Cr(III) can be easily bound by different functional groups (e.g., hydroxyl, carboxyl, amide, sulfhydryl) by a number of organic compounds (Mohan and Pittman 2006). In general, heavy metal cations can displace the native ions in metalloprotein cofactors, i.e., Ni can remove zinc from carbonic anhydrase, leading to the inactivation of the enzyme (Nieboer and Richardson 1980). They can coordinate various organic compounds resulting in the inhibition of metalloenzyme system. Heavy metal ions can also lead to replacing Ca, which stabilizes the phospholipids and pectin network in the plant surface. Therefore, we may assume that the reason for decreased leaf stiffness under Cr(III) influence is caused by the removal of Ca by Cr cations. On the contrary to trivalent Cr, Cr(VI) is a strong oxidizing agent, known for its very high toxicity to living organisms (Codd et al. 2001). In water systems, its ionic forms are anions such as chromate (CrO4 2−) or dichromate (Cr2O7 2−). The penetration of Cr(VI) through cell membranes is under the control of sulfate transporters (Kaszycki et al. 2005; Appenroth et al. 2008) and needs metabolic energy (Shanker et al. 2005). However, the penetration of Cr(VI) through cell membranes is easier than Cr(III), and the accumulation of Cr(VI) is sometimes lower than Cr(III) in some aquatic plants and microalgae (Codd et al. 2001; Frontasyeva et al. 2009). The level of Cr detected in leaves of Cr(VI)-treated C. cophocarpa was significantly lower than in Cr(III)-treated plants. These results are consistent with the previous data obtained by means of inductively coupled plasma optical emission spectrometry (ICP-OES) (Augustynowicz et al. 2013). This may result from the different sorption capacity of both species. Redox-active metallic ions such as Cr(VI) anions may lead to a number of disorders due to their ability to generate free radicals (Codd et al. 2001; Saha et al. 2011). However, according to their physicochemical properties resulting from anionic structure, they cannot replace cationic compounds.
In summary, the studies carried out using both AFM and micro-X-ray fluorescence spectroscopy showed significant differences in the properties of Callitriche cophocarpa leaves. We have observed that the distinct Cr concentrations were related to various mechanical resistance to deformations. The most important result showed the decrease in Ca content with the simultaneous increase of Cr upon exposure to the aqueous solution of Cr(III). This was accompanied by increasing the leaf’s ability to deform, which means an increase in the leaf’s elasticity. The working hypothesis assumes structural impairments due to a lack of Ca. The increase in Ca content in Cr(VI)-treated leaves resulted in the leaf’s stiffening. Since Ca ions are essential for the plant metabolism, this phenomenon is difficult to explain taking into consideration the multifaceted Ca influence on the plant (see above). Nevertheless, we have not found any reports concerning the valency-dependent influence of Cr on Ca content correlated with the leaf’s elasticity. Thus, further studies are needed to elucidate the exact mechanism of Cr(VI)-mediated increase of Ca in Callitriche leaves.
Author contribution
J. Augustynowicz was the project supervisor responsible for experimental design, collection of the plant material, preparation of samples for both AFM and XRF measurements, assistance with AFM measurements, involvement in the XRF data conversion, graphical illustration of the XRF results, interpretation of all results, writing the manuscript and correspondence with the editors and reviewers. M. Lekka was responsible for AFM measurements, calculation and interpretation of the AFM data, and graphical illustration of the AFM data, participation in writing the manuscript. P. Wróbel was responsible for XRF measurements and conversion and calculation of XRF data.
References
Appenroth KJ, Luther A, Jetschke G, Gabrys H (2008) Modification of chromate toxicity by sulphate in duckweeds (Lemnaceae). Aquat Toxicol 89:167–171
Augustynowicz J, Grosicki M, Hanus-Fajerska E, Lekka M, Waloszek A, Kołoczek H (2010) Chromium(VI) bioremediation by aquatic macrophyte Callitriche cophocarpa Sendtn. Chemosphere 79:1077–1083
Augustynowicz J, Kyzioł-Komosińska J, Smoleń S, Waloszek A (2013) Study on Cr binding capacity to Callitriche cophocarpa in an aquatic environment. Arch Environ Contam Toxicol 64:410–418
Augustynowicz J, Tokarz K, Baran A, Płachno B (2014) Phytoremediation of water polluted by Tl, Zn, Cd, and Pb with the use of macrophyte Callitriche cophocarpa. Arch Environ Contam Toxicol 66:572–581
Chandra P, Kulshreshtha K (2004) Chromium accumulation and toxicity in aquatic plants. Bot Rev 70:313–327
Codd R, Dillon CT, Levina A, Lay P (2001) Studies on genotoxicity of chromium: from the test tube to the cell. Coord Chem Rev 216–217:537–582
Dhal B, Das NN, Thatoi HN, Pandey BD (2013) Characterizing toxic Cr(VI) contamination in chromite mine overburden dump and its bacterial remediation. J Hazard Mater 260:141–149
Frontasyeva MF, Pavlov SS, Aksenova NG, Mosulishvil LM, Belokobylskii AI, Kirkesali EI, Ginturi EN, Kuchava NE (2009) Chromium interaction with blue-green microalga Spirulina platensis. J Anal Chem 64:746–749
Kaszycki P, Gabryś H, Appenroth KJ, Jaglarz A, Sędziwy S, Walczak T, Koloczek H (2005) Exogenously applied sulphate as a tool to investigate transport and reduction of chromate in the duckweed Spirodela polyrhiza. Plant Cell Environ 28:260–268
Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29:1–46
Kotaś J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Poll 107:263–283
Kyzioł-Komosińska J, Kukułka L (2008) Application of minerals co-occurring in brown coal deposits to removal of heavy metals from water and wastewater. Works and Studies 75. Polish Academy of Sciences, Zabrze (in Polish)
Mohan D, Pittman Jr ChU (2006) Activated carbon and low cost adsorbents for remediation of tri- and hexavalent chromium form water. J Hazard Mater 137:762–811
Nieboer E, Richardson DHS (1980) The replacement of the nondescript term “heavy metals” by biologically and chemically significant classification on metal ions. Environ Pollut 1:3–26
PN-EN ISO 17294-1:2007. Water quality: application of inductively coupled plasma mass spectrometry (ICP-MS). Part 1: general guidelines. Polish Committee for Standardization, Warsaw, Poland
PN-EN ISO 9963-1:2001. Water quality: determination of alkalinity. Part 1: determination of total and composite alkalinity. Polish Committee for Standardization, Warsaw, Poland
PN-ISO 9297:1994. Water quality: determination of chloride—silver nitrate titration with chromate indicator (Mohr’s method). Polish Committee for Standardization, Warsaw, Poland
Punshon T, Guerinot ML, Lanzirotti A (2009) Using synchrotron X-ray fluorescence microprobes in the study of metal homeostasis in plants. Ann Bot 103:665–672
Regulation, 9th of Nov 2011: Rozporządzenie Ministra Środowiska z dn. 9 listopada 2011 r. w sprawie sposobu klasyfikacji stanu jednolitych wód powierzchniowych oraz środowiskowych norm jakości dla substancji priorytetowych. Dziennik Ustaw nr 257, poz. 1545 (in Polish)
Saha R, Nandi R, Saha B (2011) Sources and toxicity of hexavalent chromium. J Coord Chem 64:1782–1806
Schwartz K, Mertz W (1959) Chromium(III) and the glucose tolerant factor. Arch Biochem Biophys 85:292–295
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Sperling M, Xu S, Welz B (1992) Determination of chromium(III) and chromium(VI) in water using flow injection on-line preconcentration with selective adsorption on activated alumina and flame atomic absorption spectrometric detection. Anal Chem 64:3101–3108
StatSoft, Inc. (2011) STATISTICA (data analysis software system), version 10. http://www.statsoft.com
Ubarretxena-Belandia I, Boots JW, Verheij HM, Dekker N (1998) Role of the cofactor calcium in the activation of outer membrane phospholipase A. Biochemistry 37:16011–16018
Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plan Soil 362:319–334
Vileno B, Lekka M, Sienkiewicz A, Jeney S, Stoessel G, Lekki J, Forro L (2007) Stiffness alterations of single cells induced by UV in the presence of nanoTiO2. Environ Sci Technol 41:5149–5153
Węgrzynek D, Mroczka R, Markowicz A, Chinea-Cano E, Bamford S (2008) Experimental evaluation of X-ray optics applied for microanalysis. X-Ray Spectrom 37:635–641
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Wróbel M, Czyżycki L, Furman K, Kolasiński M, Lankosz M, Mrenca A, Samek L, Węgrzynek D (2012) LabVIEW control software for scanning micro-beam X-ray fluorescence spectrometer. Talanta 93:186–192
Zayed AM, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant Soil 249:139–156
Acknowledgments
We are grateful to Dr. Anna Kołton (Institute of Plant Biology and Biotechnology, University of Agriculture in Kraków) for her help with the statistical analysis. The determination of water composition by Mr. Wiesław Knap (Department of Hydrogeology and Geological Engineering, AGH University of Science and Technology, Kraków, Poland) is highly appreciated. The study was funded by the grant DEC-2011/03/B/NZ9/00952 from the National Science Centre, Poland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klobus.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Augustynowicz, J., Lekka, M.B. & Wróbel, P.M. Mechanical properties of Callitriche cophocarpa leaves under Cr(VI)/Cr(III) influence. Acta Physiol Plant 36, 2025–2032 (2014). https://doi.org/10.1007/s11738-014-1580-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1580-2