Abstract
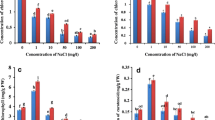
The changes in the activity of antioxidant enzymes, like superoxide dismutase, ascorbate peroxidase, catalase and glutathione reductase, and growth parameters such as length, fresh and dry weight, proline and H2O2 contents, chlorophyll fluorescence (Fv/Fm), quantum yield of PSII and the rate of lipid peroxidation in terms of malondialdehyde in leaf and root tissues of a chickpea cultivar (Cicer arietinum L. cv. Gökçe) under salt treatment were investigated. Plants were subjected to 0.1, 0.2 and 0.5 M NaCl treatments for 2 and 4 days. Compared to controls, salinity resulted in the reduction of length and of the fresh and dry weights of shoot and root tissues. Salinity caused significant (P < 0.05) changes in proline and MDA levels in leaf tissue. In general, a dose-dependent decrease was observed in H2O2 content, Fv/Fm and quantum yield of photosynthesis under salt stress. Leaf tissue extracts exhibited three activity bands, of which the higher band was identified as MnSOD and the others as FeSOD and Cu/ZnSOD. A significant enhancement was detected in the activities of Cu/ZnSOD and MnSOD isozymes in both tissues. APX and GR activities exhibited significant increases (P < 0.05) in leaf tissue under all stress treatments, whereas no significant change was observed in root tissue. The activity of CAT was significantly increased under 0.5 M NaCl stress in root tissue, while its activity was decreased in leaf tissue under 0.5 M NaCl stress for 4 days. These results suggest that CAT and SOD activities play an essential protective role against salt stress in chickpea seedlings.

Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- DW:
-
dry weight
- FW:
-
fresh weight
- GR:
-
glutathione reductase
- MDA:
-
malondialdehyde
- PAGE:
-
polyacrylamide gel electrophoresis
- PSI:
-
photosystem-I
- PSII:
-
photosystem-II
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
References
Abraham E, Rigo G, Szekely G, Nagy R, Koncz C, Szabados L (2003) Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol 51:363–372
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Bandeoğlu E, Eyidoğan F, Yücel M, Öktem HA (2004) Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul 42:69–77
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:151–155
Bernt E, Bergmeyer HU (1974) Inorganic peroxidases. In: Bergmeyer HU (eds) Methods of enzymatic analysis, Vol. 4. Academic Press, New York pp 2246–2248
Bor M, Özdemir F, Türkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77–84
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Burke JJ, Oliver MJ (1992) Differential temperature sensitivity of pea superoxide dismutases. Plant Physiol 100:1595–1598
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Dat JF, Inze D, Van Breusegem F (2001) Catalase-deficient tobacco plants: tools for in planta studies on the role of hydrogen peroxide. Redox Rep 6:37–42
Eyidoğan F, Oktem HA, Yücel M (2003) Superoxide dismutase activity in salt-stressed wheat seedlings. Acta Physiol Plant 25:263–269
Feierabend J, Dehne S (1996) Fate of the porphyrin cofactors during the light-dependent turnover of catalase and of the photosystem II reaction-center protein D1 in mature rye leaves. Planta 198:413–422
Garcia-Limones C, Hervas A, Navas-Cortes JA, Jimenez-Diaz RM, Tena M (2002) Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp.ciceris. Physiol Mol Plant Pathol 61:325–337
Hernandez JA, Campillo A, Jimenez A, Alarcon JJ, Sevilla F (1999) Response of antioxidant systems and leaf water relations to NaCl stress in pea plants. New Phytol 141:241–251
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Cal Agric Exp Sta Cir 347
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Katerji N, van Hoorn JW, Hamdy A, Mastrorilli M (2003) Salinity effect on crop development and yield, analysis of salt tolerance according to several classification methods. J Agric Water Manag 1815:1–30
Kukreja S, Nandwal AS, Kumar N, Sharma SK, Sharma SK, Unvi V, Sharma PK (2005) Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol Plant 49:305–308
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T(4). Nature 227:680–685
Lee DH, Kim YS, Lee CB (2001) The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J Plant Physiol 158:737–745
Matysik J, Alia, Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Neta ADA, Prisco JT, Filho JE, Medeiros JVR, Gomes-Filho E (2005) Hydrogen peroxide pretreatment induces salt stress acclimation in maize plants. J Plant Physiol 162:1114–1122
Ohkawa H, Ohishi N, Yagi Y (1979) Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Polle A, Eiblmeier M, Sheppard L, Murray M (1997) Responses of antioxidative enzymes to elevated CO2 in leaves of beech (Fagus sylvatica L.) seedlings grown under a range of nutrient regimes. Plant Cell Environ 20:1317–1321
Sgherri CLM, Loggini B, Puliga S, Navari-Izzo F (1994) Antioxidant system in Sporobolus stapfianus: changes in response to desiccation and rehydration. Phytochemistry 35:561–565
Singh KB, Malhotra RS, Halila MH, Knights EJ, Verma MM (1994) Current status and future strategy in breeding chickpea for resistance to biotic and abiotic stresses. Euphytica 73:137–149
Singh B, Singh BK, Kumar J, Yadav SS, Usha K (2005) Effects of salt stress on growth, nodulation, and nitrogen and carbon fixation of ten genetically diverse lines of chickpea (Cicer arietinum L.). Aust J Agric Res 56:491–495
Tramontano WA, Jouve D (1997) Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry 44:1037–1040
Wang SY, Jiao HJ, Faust M (1991) Changes in ascorbate, glutathione and related enzyme activities during thiodiazuron-induced bud break of apple. Physiol Plant 82:231–236
Welfare K, Yeo AR, Flowers TJ (2002) Effects of salinity and ozone, individually and in combination, on the growth and ion contents of two chickpea (Cicer arietinum L.) varieties. Environ Pollut 120:397–403
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, VanMontagu M, Inze D, VanCamp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. Embo J 16:4806–4816
WynJones RG, Storey R (1978) Salt stress and comparative physiology in the Gramineae 4: Comparison of salt stress in Spartina X Townsendii and 3 barley cultivars. Aust J Plant Physiol 5:839–850
Acknowledgments
The authors thank Prof. Dr. Meral Yücel and Prof. Dr. Hüseyin Avni Öktem for sharing their laboratory facilities during these experiments and for their critical review and discussion of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Bielawski.
Rights and permissions
About this article
Cite this article
Eyidogan, F., Öz, M.T. Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol Plant 29, 485–493 (2007). https://doi.org/10.1007/s11738-007-0059-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-007-0059-9