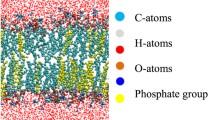
Abstract
Plasma is gaining increasing interest for cancer treatment, but the underlying mechanisms are not yet fully understood. Using computer simulations at the molecular level, we try to gain better insight in how plasma-generated reactive oxygen and nitrogen species (RONS) can penetrate through the cell membrane. Specifically, we compare the permeability of various (hydrophilic and hydrophobic) RONS across both oxidized and non-oxidized cell membranes. We also study pore formation, and how it is hampered by higher concentrations of cholesterol in the cell membrane, and we illustrate the much higher permeability of H2O2 through aquaporin channels. Both mechanisms may explain the selective cytotoxic effect of plasma towards cancer cells. Finally, we also discuss the synergistic effect of plasma-induced oxidation and electric fields towards pore formation.

Similar content being viewed by others
References
Keidar M, Walk R, Shashurin A, Srinivasan P, Sandler A, Dasgupta S, Ravi R, Guerrero-Preston R, Trink B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. British Journal of Cancer, 2011, 105(9): 1295–1301
Graves D B. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Processes and Polymers, 2014, 11(12): 1120–1127
Lu X, Naidis G V, Laroussi M, Reuter S, Graves D B, Ostrikov K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Physics Reports, 2016, 630: 1–84
Hole P S, Zabkiewicz J, Munje C, Newton Z, Pearn L, White P, Marquez N, Hills R K, Burnett A K, Tonks A, Darley R L. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood, 2013, 122(19): 3322–3330
Papadopoulos M C, Saadoun S. Key roles of aquaporins in tumor biology. Biochimica et Biophysica Acta (BBA) -Biomembranes, 2015, 1848(10): 2576–2583
Cordeiro R M. Molecular dynamics simulations of the transport of reactive oxygen species by mammalian and plant aquaporins. Biochimica et Biophysica Acta (BBA) -General Subjects, 2015, 1850(9): 1786–1794
Yan D, Talbot A, Nourmohammadi N, Sherman J H, Cheng X, Keidar M. Toward understanding the selective anticancer capacity of cold atmospheric plasma—a model based on aquaporins. Biointerphases, 2015, 10(4): 040801
Wong-Ekkabut J, Xu Z, Triampo W, Tang I M, Tieleman D P, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophysical Journal, 2007, 93(12): 4225–4236
Beranova L, Cwiklik L, Jurkiewicz P, Hof M, Jungwirth P. Oxidation changes physical properties of phospholipid bilayers: Fluorescence spectroscopy and molecular simulations. Langmuir, 2010, 26(9): 6140–6144
Cwiklik L, Jungwirth P. Massive oxidation of phospholipid membranes leads to pore creation and bilayer disintegration. Chemical Physics Letters, 2010, 486(4–6): 99–103
Van der Paal J, Neyts E C, Verlackt C C W, Bogaerts A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chemical Science (Cambridge), 2016, 7(1): 489–498
Van der Paal J, Verheyen C, Neyts E C, Bogaerts A. Hampering effect of cholesterol on the permeation of reactive oxygen species through phospholipids bilayer: Possible explanation for plasma cancer selectivity. Scientific Reports, 2017, 7(1): 39526
Svarnas P, Matrali S H, Gazeli K, Antimisiaris S G. Assessment of atmospheric-pressure guided streamer (plasma bullet) influence on liposomes with different composition and physicochemical properties. Plasma Processes and Polymers, 2015, 12(7): 655–665
Hirst A M, Frame F M, Arya M, Maitland N J, O’Connell D. Low temperature plasmas as emerging cancer therapeutics: The state of play and thoughts for the future. Tumour Biology, 2016, 37(6): 7021–7031
Robert E, Darny T, Dozias S, Iseni S, Pouvesle J M. New insights on the propagation of pulsed atmospheric plasma streams: From single jet to multi jet arrays. Physics of Plasmas, 2015, 22(12): 122007
Begum A, Laroussi M, Pervez M R. Atmospheric pressure He-air plasma jet: Breakdown process and propagation phenomenon. AIP Advances, 2013, 3(6): 062117
Weaver J C, Smith K C, Esser A T, Son R S, Gowrishankar T. A brief overview of electroporation pulse strength-duration space: A region where additional intracellular effects are expected. Bioelectrochemistry (Amsterdam, Netherlands), 2012, 87: 236–243
Vernier P T, Ziegler M J. Nanosecond field alignment of head group and water dipoles in electroporating phospholipid bilayers. Journal of Physical Chemistry B, 2007, 111(45): 12993–12996
Casciola M, Tarek M. A molecular insight into the electro-transfer of small molecules through electropores driven by electric fields. Biochimica et Biophysica Acta (BBA) -Biomembranes, 2016, 1858 (10): 2278–2289
Marrink S J, de Vries A H, Tieleman D P. Lipids on the move: simulations of membrane pores, domains, stalks and curves. Biochimica et Biophysica Acta (BBA) -Biomembranes, 2009, 1788(1): 149–168
Tai WY, Yang Y C, Lin H J, Huang C P, Cheng Y L, Chan MF, Yen H L, Liau I. Interplay between structure and fluidity of model lipid membranes under oxidative attack. Journal of Physical Chemistry B, 2010, 114(47): 15642–15649
Grzelinska E, Bartosz G, Gwozdzinski K, Leyko W. A spin-label study of the effect of gamma radiation on erythrocyte membrane. Influence of lipid peroxidation on membrane structure. International Journal of Radiation Biology, 1979, 36: 325–334
Wratten M L, Van Ginkel G, Van’t Veld A A, Bekker A, Van Faassen E E, Sevanian A. Structural and dynamic effects of oxidatively modified phospholipids in unsaturated lipid membranes. Biochemistry, 1992, 31(44): 10901–10907
Chen J J, Yu B P. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radical Biology & Medicine, 1994, 17(5): 411–418
Richter C. Biophysical consequences of lipid peroxidation in membranes. Chemistry and Physics of Lipids, 1987, 44(2–4): 175–189
Mason R P, Walter M F, Mason P E. Effect of oxidative stress on membrane structure: Small-angle X-ray diffraction analysis. Free Radical Biology & Medicine, 1997, 23(3): 419–425
Szili E J, Hong S H, Short R D. On the effect of serum on the transport of reactive oxygen species across phospholipid membranes. Biointerphases, 2015, 10(2): 029511
Lee E H, Hsin J, Sotomayor M, Comellas G, Schulten K. Discovery through the computational microscope. Structure (London, England), 2009, 17(10): 1295–1306
Cordeiro R M. Reactive oxygen species at phospholipid bilayers: Distribution, mobility and permeation. Biochimica et Biophysica Acta (BBA) -Biomembranes, 2014, 1838(1): 438–444
Miotto R, Costa E B, Trellese E B, Neto A J P, Baptista M S, Ferraz A C, Cordeiro R M. Biomembranes under oxidative stress, Insights from molecular dynamics simulations. In: Tran Q N, Arabnia H R, eds. Emerging Trends in Applications and Infrastructures for Computational Biology, Bioinformatics, and Systems Biology: Systems and Applications. Amsterdam: Elsevier, 2016, 197–211
Yusupov M, Van der Paal J, Neyts E C, Bogaerts A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochimica et Biophysica Acta (BBA) -General, 2017, 1861: 839–847
Yusupov M, Wende K, Kupsch S, Neyts E C, Reuter S, Bogaerts A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Scientific Reports, 2017, 7(1): 5761
Yusupov M, Yan D, Cordeiro R M, Bogaerts A. Atomic scale simulation of H2O2 permeation through aquaporin: Toward the understanding of plasma-cancer treatment. Journal of Physics. D, Applied Physics, 2018, 51(12): 125401
Razzokov J, Yusupov M, Cordeiro R M, Bogaerts A. Atomic scale understanding of the permeation of plasma species across native and oxidized membranes. Journal of Physics. D, Applied Physics, 2018, 51(36): 365203
Bogaerts A, Khosravian N, Van der Paal J, Verlackt C C W, Yusupov M, Kamaraj B, Neyts E C. Multi-level molecular modeling for plasma medicine. Journal of Physics. D, Applied Physics, 2016, 49(5): 054002
Elstner M, Porezag D, Jungnickel G, Elsner J, Haugk M, Frauenheim Th, Suhai S, Seifert G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Physical Review. B, 1998, 58(11): 7260–7268
Khosravian N, Kamaraj B, Neyts E C, Bogaerts A. Structural modification of P-glycoprotein induced by OH radicals: Insights from atomistic simulations. Scientific Reports, 2016, 6(1): 19466
Verlackt C C W, Van Boxem W, Dewaele D, Lemière F, Sobott F, Benedikt J, Neyts E C, Bogaerts A. Mechanisms of peptide oxidation by hydroxyl radicals: Insight at the molecular scale. Journal of Physical Chemistry C, 2017, 121(10): 5787–5799
Verlackt C C W, Neyts E C, Bogaerts A. Atomic scale behavior of oxygen-based radicals in water. Journal of Physics. D, Applied Physics, 2017, 50(11): 11LT01
Brenner D W. Empirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond films. Physical Review. B, 1990, 42(15): 9458–9471
van Duin A C T, Dasgupta S, Lorant F, Goddard W A, Reax F F. A reactive force field for hydrocarbons. Journal of Physical Chemistry A, 2001, 105(41): 9396–9409
Yusupov M, Neyts E C, Khalilov U, Snoeckx R, van Duin A C T, Bogaerts A. Atomic scale simulations of plasma species interacting with bacterial cell walls. New Journal of Physics, 2012, 14(9): 093043
Yusupov M, Bogaerts A, Huygh S, Snoeckx S, van Duin A C T, Neyts E C. Plasma-induced destruction of bacterial cell wall components: A reactive molecular dynamics simulation. Journal of Physical Chemistry C, 2013, 117(11): 5993–5998
Yusupov M, Neyts E C, Verlackt C C, Khalilov U, van Duin A C T, Bogaerts A. Inactivation of the endotoxic biomolecule lipid A by oxygen plasma species: A reactive molecular dynamics study. Plasma Processes and Polymers, 2015, 12(2): 162–171
Babaeva N Y, Ning N, Graves D B, Kushner M J. Ion activation energy delivered to wounds by atmospheric pressure dielectricbarrier discharges: Sputtering of lipid-like surfaces. Journal of Physics. D, Applied Physics, 2012, 45(11): 115203
Van der Paal J, Aernouts S, van Duin A C T, Neyts E C, Bogaerts A. Interaction of O and OH radicals with a simple model system for lipids in the skin barrier: A reactive molecular dynamics simulation for plasma medicine. Journal of Physics. D, Applied Physics, 2013, 46(39): 395201
Van der Paal J, Verlackt C C, Yusupov M, Neyts E C, Bogaerts A. Structural modification of the skin barrier by OH radicals: A reactive molecular dynamics study for plasma medicine. Journal of Physics. D, Applied Physics, 2015, 48(15): 155202
Abolfath R M, Biswas P K, Rajnarayanam R, Brabec T, Kodym R, Papiez L. Multiscale QM/MM molecular dynamics study on the first steps of guanine damage by free hydroxyl radicals in solution. Journal of Physical Chemistry A, 2012, 116(15): 3940–3945
Verlackt C C M, Neyts E C, Jacob T, Fantauzzi D, Golkaram M, Shin Y K, van Duin A C T, Bogaerts A. Atomic-scale insight in the interactions between hydroxyl radicals and DNA in solution using the ReaxFF reactive force field. New Journal of Physics, 2015, 17 (10): 103005
Yusupov M, Neyts E C, Simon P, Bergiyorov G, Snoeckx R, van Duin A C T, Bogaerts A. Reactive molecular dynamics simulations of oxygen species in a liquid water layer of interest for plasma medicine. Journal of Physics. D, Applied Physics, 2014, 47(2): 025205
Khosravian N, Bogaerts A, Huygh S, Yusupov M, Neyts E C. How do plasma-generated OH radicals react with biofilm components? Insights from atomic scale simulations. Biointerphases, 2015, 10(2): 029501
Berger O, Edholm O, Jähnig F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophysical Journal, 1997, 72(5): 2002–2013
Cornell W D, Cieplak P, Bayly C I, Gould I R, Merz K M Jr, Ferguson D M, Spellmeyer D C, Fox T, Caldwell J W, Kollman P A. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. Journal of the American Chemical Society, 1995, 117(19): 5179–5197
Yu W, He X, Vanommeslaeghe K, MacKerell A D Jr. Extension of the CHARMM general force field to sulfonyl-containing compounds and its utility in biomolecular simulations. Journal of Computational Chemistry, 2012, 33(31): 2451–2468
van Gunsteren W F, Berendsen H J C. Groningen Molecular Simulation (GROMOS) Library Manual. Groningen, The Netherlands: Biomos, 1987, 1–221
Marrink S J, Risselada H J, Yefimov S, Tieleman D P, De Vries A H. The MARTINI force field: Coarse grained model for biomolecular simulations. Journal of Physical Chemistry B, 2007, 111(27): 7812–7824
Åqvist J, Warshel A. Simulation of enzyme reactions using valence bond force fields and other hybrid quantum/classical approaches. Chemical Reviews, 1993, 93(7): 2523–2544
Neyts E C, Yusupov M, Verlackt C C, Bogaerts A. Computer simulations of plasma-biomolecule and plasma-tissue interactions for a better insight in plasma medicine. Journal of Physics. D, Applied Physics, 2014, 47(29): 293001
Möller M N, Li Q, Lancaster J R Jr, Denicola A. Acceleration of nitric oxide autoxidation and nitrosation by membranes. IUBMB Life, 2007, 59(4): 243–248
Subczynski W K, Lomnicka M, Hyde J S. Permeability of nitric oxide through lipid bilayer membranes. Free Radical Research, 1996, 24(5): 343–349
Shinitzky M. Membrane fluidity in malignancy adversative and recuperative. Biochimica et Biophysica Acta (BBA) -Revue Canadienne, 1984, 738: 251–261
Reis A, Domingues M R M, Amado F M L, Ferrer-Correia A J V, Domingues P. Separation of peroxidation products of diacylphosphatidylcholines by reversed-phase liquid chromatographymass spectrometry. Biomedical Chromatography, 2005, 19(2): 129–137
Haluska C K, Baptista M S, Fernades A U, Schroder A P, Marques C M, Itri R. Photo-activated phase separation in giant vesicles made from different lipid mixture. Biochimica et Biophysica Acta (BBA) -Biomembranes, 2012, 1818: 666–672
Lackmann J W, Schneider S, Edengeiser E, Jarzina F, Brinckmann S, Steinborn E, Havenith M, Benedikt J, Bandow J E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. Journal of the Royal Society, Interface, 2013, 10(89): 20130591
Chung T Y, Ning N, Chu J W, Graves D B, Bartis E, Seog J, Oehrlein G S. Plasma deactivation of endotoxic biomolecules: Vacuum ultraviolet photon and radical beam effects on Lipid A. Plasma Processes and Polymers, 2013, 10(2): 167–180
Bartis E A J, Graves D B, Seog J, Oehrlein G S. Atmospheric pressure plasma treatment of lipopolysaccharide in a controlled environment. Journal of Physics. D, Applied Physics, 2013, 46(31): 312002 doi:10.1088/0022-3727/46/31/312002
Bartis E A J, Barrett C, Chung T Y, Ning N, Chu J W, Graves D B, Seog J, Oehrlein G S. Deactivation of lipopolysaccharide by Ar and H2 inductively coupled low-pressure plasma. Journal of Physics. D, Applied Physics, 2014, 47(4): 045202
Park J H, Kumar N, Park D H, Yusupov M, Neyts E C, Verlackt C C W, Bogaerts A, Kang M H, Uhm H S, Choi E H, et al. A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nano-second pulsed plasma. Scientific Reports, 2015, 5(1): 13849
Marschewski M, Hirschberg J, Omairi T, Hofft O, Viol W, Emmert S, Maus-Friedrichs W. Electron spectroscopic analysis of the human lipid skin barrier: Cold atmospheric plasma-induced changes in lipid composition. Experimental Dermatology, 2012, 21 (12): 921–925
Takai E, Kitamura T, Kuwabara J, Ikawa S, Yoshizawa S, Shiraki K, Kawasaki H, Arakawa R, Kitano K. Chemical modification of amino acids by atmospheric-pressure Cold plasma in aqueous solution. Journal of Physics. D, Applied Physics, 2014, 47(28): 285403
Madugundu G S, Cadet J, Wagner J R. Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic Acids Research, 2014, 42(11): 7450–7460
Hong S H, Szili E J, Jenkins A T A, Short R D. Ionized gas (plasma) delivery of reactive oxygen species (ROS) into artificial cells. Journal of Physics. D, Applied Physics, 2014, 47(36): 362001
Szili E J, Bradley J W, Short R D. A ‘tissue model’ to study the plasma delivery of reactive oxygen species. Journal of Physics. D, Applied Physics, 2014, 47(15): 152002
Hammer M U, Forbrig E, Kupsch S, Weltmann K D, Reuter S. Influence of plasma treatment on the structure and function of lipids. Plasma Medicine, 2013, 3(1–2): 97–114
Acknowledgements
We acknowledge financial support from the Research Foundation–Flanders (FWO; Grant Nos. 1200216N and 11U5416N). The computational work was carried out using the Turing HPC infrastructure at the CalcUA core facility of the Universiteit Antwerpen (UA), a division of the Flemish Supercomputer Center VSC, funded by the Hercules Foundation, the Flemish Government (department EWI) and the UA. We are also very thankful to R. Cordeiro for the very interesting discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bogaerts, A., Yusupov, M., Razzokov, J. et al. Plasma for cancer treatment: How can RONS penetrate through the cell membrane? Answers from computer modeling. Front. Chem. Sci. Eng. 13, 253–263 (2019). https://doi.org/10.1007/s11705-018-1786-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-018-1786-8