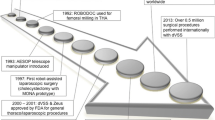
Abstract
Robotic surgery represents a milestone in surgical procedures, offering advantages such as less invasive methods, elimination of tremors, scaled motion, and 3D visualization. This in-depth analysis explores the complex biochemical effects of robotic methods. The use of pneumoperitoneum and steep Trendelenburg positioning can decrease pulmonary compliance and splanchnic perfusion while increasing hypercarbia. However, robotic surgery reduces surgical stress and inflammation by minimizing tissue trauma. This contributes to faster recovery but may limit immune function. Robotic procedures also limit ischemia–reperfusion injury and oxidative damage compared to open surgery. They also help preserve native antioxidant defenses and coagulation. In a clinical setting, robotic procedures reduce blood loss, pain, complications, and length of stay compared to traditional procedures. However, risks remain, including device failure, the need for conversion to open surgery and increased costs. On the oncology side, there is still debate about margins, recurrence, and long-term survival. The advent of advanced technologies, such as intraoperative biosensors, localized drug delivery systems, and the incorporation of artificial intelligence, may further improve the efficiency of robotic surgery. However, ethical dilemmas regarding patient consent, privacy, access, and regulation of this disruptive innovation need to be addressed. Overall, this review sheds light on the complex biochemical implications of robotic surgery and highlights areas that require additional mechanistic investigation. It presents a comprehensive approach to responsibly maximize the potential of robotic surgery to improve patient outcomes, integrating technical skill with careful consideration of physiological and ethical issues.


Similar content being viewed by others
Data availability
Data availability correspondence should be addressed to noorazarian_a@khoyums.ac.ir.
References
Bliznakova K, Kolev N, Zlatarov A, Kalinov T, Georgiev T (2023) Feasibility and safety of robotic-assisted surgery for rectal cancer: short-term outcomes of a pilot study with da Vinci Xi platform during COVID-19. Chirurgia (Bucur) 118:27–38. https://doi.org/10.21614/chirurgia.2688
Kerray F, Yule S (2021) Rise of the machines: human factors and training for robotic-assisted surgery. BMJ Surg Interv Health Technol 3:e000100. https://doi.org/10.1136/bmjsit-2021-000100
Villalobos R, Maestre Y, González Barranquero A, Olsina J (2022) V-038 inguinal tep with articulated instruments: beyond conventional laparoscopy, closer to robotic surgery. Br J Surg. https://doi.org/10.1093/bjs/znac308.290
Kumar P, Talele S, Deshpande S, Ghyar R, Rout S, Ravi B (2023) Design, analysis and experimental validation of a novel 7-degrees of freedom instrument for laparoscopic surgeries. Ann Biomed Eng 51:751–770. https://doi.org/10.1007/s10439-022-03086-w
Kayani B, Haddad FS (2019) Robotic total knee arthroplasty: clinical outcomes and directions for future research. Bone Joint Res 8:438–442. https://doi.org/10.1302/2046-3758.810.BJR-2019-0175
Marino MV, Shabat G, Gulotta G, Komorowski AL (2018) From illusion to reality: a brief history of robotic surgery. Surg Innov 25:291–296. https://doi.org/10.1177/1553350618771417
Chan F (2018) Robotic-assisted surgical procedures are the future of gynaecology in Australasia. Aust N Z J Obstet Gynaecol 58:371–374. https://doi.org/10.1111/ajo.12819
Danesh H, Rahmati J, Mahdieh M, Hemadi SM, Bahmani A (2022) Medical and chemical evaluation of robotic surgery methods; a review study. Rom J Mil Med 125:542–551. https://doi.org/10.55453/rjmm.2022.125.4.2
Rivas-Lopez R, Sandoval-Garcia-Travesi FA (2020) Robotic surgery in gynecology: review of literature. Cir Cir 88:107–116. https://doi.org/10.24875/CIRU.18000636
Krzystek-Korpacka M, Zawadzki M, Lewandowska P, Szufnarowski K, Bednarz-Misa I, Jacyna K et al (2019) Distinct chemokine dynamics in early postoperative period after open and robotic colorectal surgery. J Clin Med. https://doi.org/10.3390/jcm8060879
Mazzella A, Casiraghi M, Galetta D, Cara A, Maisonneuve P, Petrella F et al (2023) How much stress does a surgeon endure? The effects of the robotic approach on the autonomic nervous system of a surgeon in the modern era of thoracic surgery. Cancers (Basel). https://doi.org/10.3390/cancers15041207
Cepolina F, Razzoli RP (2022) An introductory review of robotically assisted surgical systems. Int J Med Robot 18:e2409. https://doi.org/10.1002/rcs.2409
Zhang QB, Zhao C, Luo XM (2011) Ergonomic analysis of the master of minimally invasive surgical robot. Adv Mater Res 418–420:2018–2023. https://doi.org/10.4028/www.scientific.net/AMR.418-420.2018
Yang Y, Song L, Huang J, Cheng X, Luo Q (2021) A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) surgical system in the treatment of early-stage lung cancer. Transl Lung Cancer Res 10:1571–1575. https://doi.org/10.21037/tlcr-21-207
Stefano GB (2017) Robotic surgery: fast forward to telemedicine. Med Sci Monit 23:1856. https://doi.org/10.12659/msm.904666
Kumar S (2016) Open versus robotic prostatectomy. Indian J Urol 32:253–254. https://doi.org/10.4103/0970-1591.191233
Brassetti A, Ragusa A, Tedesco F, Prata F, Cacciatore L, Iannuzzi A et al (2023) Robotic surgery in urology: history from PROBOT® to HUGOTM. Sensors. https://doi.org/10.3390/s23167104
Goh EZ, Ali T (2022) Robotic surgery: an evolution in practice. J Surg Prot Res Methodol. https://doi.org/10.1093/jsprm/snac003
Chen J, Xu H, Lin S, He S, Tang K, Xiao Z et al (2022) Robot-assisted pyeloplasty and laparoscopic pyeloplasty in children: a comparison of single-port-plus-one and multiport surgery. Front Pediatr 10:957790. https://doi.org/10.3389/fped.2022.957790
Yagisawa T, Takagi T, Yoshida K, Hata K, Iizuka J, Muromiya Y et al (2022) Surgical outcomes of robot-assisted laparoscopic partial nephrectomy for cystic renal cell carcinoma. J Robot Surg 16:649–654. https://doi.org/10.1007/s11701-021-01292-7
Packiam VT, Barashi NS, Shalhav AL (2018) Robot-assisted laparoscopic adrenalectomy. J Endourol 32:S82–S87. https://doi.org/10.1089/end.2017.0721
Hashizume M (2005) Robot-assisted surgery. Geka Gakkai Zasshi 106:689–93
Grimminger PP, Hadzijusufovic E, Ruurda JP, Lang H, van Hillegersberg R (2018) The da Vinci Xi robotic four-arm approach for robotic-assisted minimally invasive esophagectomy. Thorac Cardiovasc Surg 66:407–409. https://doi.org/10.1055/s-0038-1636933
Rodríguez RAC, Noguera RJS (2021) New horizons in robotic surgery: da vinci begins to compete. Revista Urol Colomb Colomb Urol J 30:e153–e154. https://doi.org/10.1055/s-0041-1737013
Pathirana S, Kam P (2018) Anaesthetic issues in robotic-assisted minimally invasive surgery. Anaesth Intensive Care 46:25–35. https://doi.org/10.1177/0310057X1804600105
Wang H, Xu Z, Wu A, Dong Y, Zhang Y, Yue Y et al (2015) 2-Deoxy-D-glucose enhances anesthetic effects in mice. Anesth Analg 120:312–319. https://doi.org/10.1213/ANE.0000000000000520
Luz AL, Lagido C, Hirschey MD, Meyer JN (2016) In vivo determination of mitochondrial function using luciferase-expressing Caenorhabditis elegans: contribution of oxidative phosphorylation, glycolysis, and fatty acid oxidation to toxicant-induced dysfunction. Curr Protoc Toxicol. https://doi.org/10.1002/cptx.10
Sheridan RL, Prelack K, Szyfelbein SK (1997) Neuromuscular blockade does not decrease oxygen consumption or energy expenditure beyond sedation in the mechanically ventilated child. J Intensive Care Med 12:321–323. https://doi.org/10.1177/088506669701200606
Nair AS, Christopher A, Kotthapalli KK, Mantha SP (2021) Laparoscopic surgeries and carbon dioxide pneumoperitoneum during COVID-19 pandemic: problems and solutions. Med Gas Res 11:46. https://doi.org/10.4103/2045-9912.310060
Badani KK, Shapiro EY, Berg WT, Kaufman S, Bergman A, Wambi C et al (2013) A pilot study of laparoscopic Doppler ultrasound probe to map arterial vascular flow within the neurovascular bundle during robot-assisted radical prostatectomy. Prostate Cancer 2013:810715. https://doi.org/10.1155/2013/810715
Aditianingsih D, Mochtar CA, Lydia A, Siregar NC, Margyaningsih NI, Madjid AS et al (2020) Effects of low versus standard pressure pneumoperitoneum on renal syndecan-1 shedding and VEGF receptor-2 expression in living-donor nephrectomy: a randomized controlled study. BMC Anesthesiol 20:37. https://doi.org/10.1186/s12871-020-0956-7
Johnson DJ, Brooks DC, Pressler VM, Hulton NR, Colpoys MF, Smith RJ et al (1986) Hypothermic anesthesia attenuates postoperative proteolysis. Ann Surg 204:419–429. https://doi.org/10.1097/00000658-198610000-00010
Slavin M, Goldstein A, Raguan B, Rudnicki Y, Avital S, White I (2022) Postoperative CRP levels can rule out anastomotic leaks in Crohn’s disease patients. J Pers Med. https://doi.org/10.3390/jpm12010054
Preisser F, Nazzani S, Mazzone E, Marchioni M, Bandini M, Tian Z et al (2019) Comparison of open versus robotically assisted cytoreductive radical prostatectomy for metastatic prostate cancer. Clin Genitourin Cancer 17:e939–e945. https://doi.org/10.1016/j.clgc.2019.05.022
Fan S, Zhong JL, Chen WX, Chen WL, Li QX, Wang YY et al (2017) Postoperative immune response and surgical stress in selective neck dissection: comparison between endoscopically assisted dissection and open techniques in cT1-2N0 oral squamous cell carcinoma. J Craniomaxillofac Surg 45:1112–1116. https://doi.org/10.1016/j.jcms.2016.11.021
He L, Qing F, Li M, Lan D (2021) Effects of laparoscopic and traditional open surgery on the levels of IL-6, TNF-alpha, and Gal-3 in patients with thyroid cancer. Gland Surg 10:1085–1092. https://doi.org/10.21037/gs-21-60
Mueller AA, Kalak N, Schwenzer-Zimmerer K, Holsboer-Trachsler E, Brand S (2014) Cortisol levels and sleep patterns in infants with orofacial clefts undergoing surgery. Neuropsychiatr Dis Treat 10:1965–1972. https://doi.org/10.2147/NDT.S71785
Fleszar MG, Fortuna P, Zawadzki M, Hodurek P, Bednarz-Misa I, Witkiewicz W et al (2021) Sex, type of surgery, and surgical site infections are associated with perioperative cortisol in colorectal cancer patients. J Clin Med. https://doi.org/10.3390/jcm10040589
Landesberg G, London MJ (2016) The enigma of postoperative troponin elevation. Anesth Analg 123:5–7. https://doi.org/10.1213/ANE.0000000000001336
Sjodahl R, Davidson T, Aldman A, Lennmarken C, Kammerlind AS, Gustavsson E, et al. (2022) [Robotic-assisted pelvic and renal surgery—an overview]. Lakartidningen. p119
Neidert MC, Losa M, Regli L, Sarnthein J (2015) Elevated serum creatine kinase after neurosurgeries in lateral position with intraoperative neurophysiological monitoring is associated with OP duration. BMI and age Clin Neurophysiol 126:2026–2032. https://doi.org/10.1016/j.clinph.2014.12.019
Tsuchiya Y, Munakata S, Tsukamoto R, Okazawa Y, Mizukoshi K, Sugimoto K et al (2020) Creatine kinase elevation after robotic surgery for rectal cancer due to a prolonged lithotomy position. BMC Surg 20:136. https://doi.org/10.1186/s12893-020-00771-2
Sahutoglu C, Yasar A, Kocabas S, Askar FZ, Ayik MF, Atay Y (2018) Correlation between serum lactate levels and outcome in pediatric patients undergoing congenital heart surgery. Turk Gogus Kalp Damar Cerrahisi Derg 26:375–385. https://doi.org/10.5606/tgkdc.dergisi.2018.15791
Kumar L, Kumar K, Sandhya S, Koshy DM, Ramamurthi KP, Rajan S (2020) Effect of liberal versus restrictive fluid therapy on intraoperative lactate levels in robot- assisted colorectal surgery. Indian J Anaesth 64:599–604. https://doi.org/10.4103/ija.IJA_401_20
Soltanizadeh S, Jensen KK, Nordklint AK, Jorgensen HL, Jorgensen LN (2023) Even minor alteration of plasma creatinine after open abdominal surgery is associated with 30-day mortality: A single-centre cohort study. J Visc Surg 160:19–26. https://doi.org/10.1016/j.jviscsurg.2021.10.008
Windisch OL, Matter M, Pascual M, Sun P, Benamran D, Buhler L et al (2022) Robotic versus hand-assisted laparoscopic living donor nephrectomy: comparison of two minimally invasive techniques in kidney transplantation. J Robot Surg 16:1471–1481. https://doi.org/10.1007/s11701-022-01393-x
Minami E, Ito S, Sugiura T, Fujita Y, Sasano H, Sobue K (2014) Markedly elevated procalcitonin in early postoperative period in pediatric open heart surgery: a prospective cohort study. J Intensive Care 2:38. https://doi.org/10.1186/2052-0492-2-38
Cave J, Clarke S (2018) Paediatric robotic surgery. Ann R Coll Surg Engl 100:18–21. https://doi.org/10.1308/rcsann.supp2.18
Djordjevic A, Kotnik P, Horvat D, Knez Z, Antonic M (2020) Pharmacodynamics of malondialdehyde as indirect oxidative stress marker after arrested-heart cardiopulmonary bypass surgery. Biomed Pharmacother 132:110877. https://doi.org/10.1016/j.biopha.2020.110877
Kozlik J, Przybylowska J, Mikrut K, Zukiewicz-Sobczak WA, Zwolinski J, Piatek J et al (2015) Selected oxidative stress markers in gynecological laparoscopy. Wideochir Inne Tech Maloinwazyjne 10:92–100. https://doi.org/10.5114/wiitm.2014.47449
Koźlik J, Przybyłowska J, Mikrut K, Żukiewicz-Sobczak WA, Zwoliński J, Piątek J et al (2015) Selected oxidative stress markers in gynecological laparoscopy. Videosurg Other Miniinvasive Tech 1:92–100. https://doi.org/10.5114/wiitm.2014.47449
Burton D, Nicholson G, Hall G (2004) Endocrine and metabolic response to surgery. Cont Educ Anaesth Crit Care Pain 4:144–147. https://doi.org/10.1093/bjaceaccp/mkh040
Dronkers J, Witteman B, van Meeteren N (2016) Surgery and functional mobility: doing the right thing at the right time. Tech Coloproctol 20:339–341. https://doi.org/10.1007/s10151-016-1487-6
Narra GR, Aparna SM, Miraz M, Santhosh S (2015) Comparison of surgical stress response under general anaesthesia in open laparotomy vs. laparoscopic abdominal surgeries. J Evol Med Dental Sci. 4:15125–15133. https://doi.org/10.14260/jemds/2015/2148
Azemati S, Savai M, Khosravi MB, Allahyari E, Jahanmiri F (2013) Combination of remifentanil with isoflurane or propofol: effect on the surgical stress response. Acta Anaesthesiol Belg 64:25–31
Hernández-Avalos I, Flores-Gasca E, Mota-Rojas D, Casas-Alvarado A, Miranda-Cortés AE, Domínguez-Oliva A (2021) Neurobiology of anesthetic-surgical stress and induced behavioral changes in dogs and cats: a review. Veterinary World 14:393–404. https://doi.org/10.14202/vetworld.2021.393-404
Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J (2019) The role of interleukin-1 in general pathology. Inflamm Regen 39:12. https://doi.org/10.1186/s41232-019-0101-5
Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F et al (2022) COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 19:92. https://doi.org/10.1186/s12985-022-01814-1
Anderson HL, Stryjewska B, Boyanton BL, Schwartz MR (1981) Review of the literature. Acta Anaesthesiol Scand 25:8–16. https://doi.org/10.1111/j.1399-6576.1981.tb01707.x
Funahashi H, Mizuno S (1985) Pituitary-adrenocortical response to surgical stress in male patients. Nihon Geka Gakkai Zasshi. 86:240–250
Hirokawa K, Miwa M, Taniguchi T, Tsuchiya M, Kawakami N (2016) Moderating effects of salivary testosterone levels on associations between job demand and psychological stress response in Japanese medical workers. Ind Health 54:194–203. https://doi.org/10.2486/indhealth.2015-0113
Pucheril D, Fletcher SA, Chen X, Friedlander DF, Cole AP, Krimphove MJ et al (2021) Workplace absenteeism amongst patients undergoing open vs. robotic radical prostatectomy, hysterectomy, and partial colectomy. Surg Endosc 35:1644–1650. https://doi.org/10.1007/s00464-020-07547-y
Vlot J (2019) Invited brief commentary on IUVS -2017-0216. J Invest Surg 32:61–62. https://doi.org/10.1080/08941939.2017.1383537
Todd LA, Vigersky RA (2021) Evaluating perioperative glycemic control of non-cardiac surgical patients with diabetes. Mil Med 186:e867–e872. https://doi.org/10.1093/milmed/usaa467
Pick A, Baralli JD, Sandhu H, Khorzad R (2020) 2231-PUB: perioperative glucose control protocol: hip and knee arthroplasty. Diabetes. https://doi.org/10.2337/db20-2231-PUB
Behrenbruch C, Shembrey C, Paquet-Fifield S, Molck C, Cho HJ, Michael M et al (2018) Surgical stress response and promotion of metastasis in colorectal cancer: a complex and heterogeneous process. Clin Exp Metastasis 35:333–345. https://doi.org/10.1007/s10585-018-9873-2
Rossano F (2019) Complications of robotic surgery in oncological gynecology: the experience of the Brazilian National Institute of Cancer. J Gynecol Res Obstetr. https://doi.org/10.17352/jgro.000065
Sies H (2020) Oxidative stress: concept and some practical aspects. Antioxidants (Basel). https://doi.org/10.3390/antiox9090852
Ou W, Liang Y, Qin Y, Wu W, Xie M, Zhang Y et al (2021) Hypoxic acclimation improves cardiac redox homeostasis and protects heart against ischemia-reperfusion injury through upregulation of O-GlcNAcylation. Redox Biol 43:101994. https://doi.org/10.1016/j.redox.2021.101994
Sugita J, Fujiu K (2018) Systemic inflammatory stress response during cardiac surgery. Int Heart J 59:457–459. https://doi.org/10.1536/ihj.18-210
Rutkowski R, Pancewicz SA, Rutkowski K, Rutkowska J (2007) Reactive oxygen and nitrogen species in inflammatory process. Pol Merkur Lekarski 23:131–136
Sodha S, Nazarian S, Adshead JM, Vasdev N, Mohan SG (2016) Effect of pneumoperitoneum on renal function and physiology in patients undergoing robotic renal surgery. Curr Urol 9:1–4. https://doi.org/10.1159/000442842
Gielen CE, Pignez Y, Swaelens C (2023) Two cases of vascular complications after urologic robotic surgery. Acta Chir Belg 123:333–336. https://doi.org/10.1080/00015458.2021.2021357
Gokmen Karasu AF, Kiran G, Sanlikan F (2022) Intraoperative complications and conversion to laparatomy in gynecologic robotic surgery. J Invest Surg 35:912–915. https://doi.org/10.1080/08941939.2021.1949411
Bastopcu M, Senay S, Gullu AU, Kocyigit M, Alhan C (2022) Percutaneous cannulation for cardiopulmonary bypass in robotic mitral valve surgery with zero groin complications. J Card Surg 37:280–284. https://doi.org/10.1111/jocs.16090
Pechan I, Danova K, Olejarova I, Halcak L, Rendekova V, Fabian J (2003) Oxidative stress and antioxidant defense systems in patients after heart transplantation. Wien Klin Wochenschr 115:648–651. https://doi.org/10.1007/BF03040470
Tokusoglu O (2019) Robotic approaches help maintain higher levels of key antioxidants like glutathione, superoxide dismutase, and catalase compared to open procedures. Food Health Technol Innov. 2:193–196
Hubner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schafer M (2016) Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract 2016:8743187. https://doi.org/10.1155/2016/8743187
Breuer JP, Heymann CV, Spies C (2009) Perioperative Ernährung—metabolische Konditionierung. Aktuelle Ernährungsmedizin 34:107–113. https://doi.org/10.1055/s-0028-1090143
Mila-Kierzenkowska C, Wozniak A, Drewa T, Wozniak B, Szpinda M, Krzyzynska-Malinowska E et al (2013) Effects of open versus laparoscopic nephrectomy techniques on oxidative stress markers in patients with renal cell carcinoma. Oxid Med Cell Longev 2013:438321. https://doi.org/10.1155/2013/438321
Arsalani-Zadeh R, Ullah S, Khan S, MacFie J (2011) Oxidative stress in laparoscopic versus open abdominal surgery: a systematic review. J Surg Res 169:e59-68. https://doi.org/10.1016/j.jss.2011.01.038
Jaradeh M, Curran B, Poulikidis K, Rodrigues A, Jeske W, Abdelsattar ZM et al (2022) Inflammatory cytokines in robot-assisted thoracic surgery versus video-assisted thoracic surgery. J Thorac Dis 14:2000–2010. https://doi.org/10.21037/jtd-21-1820
Pilka R, Marek R, Adam T, Kudela M, Ondrova D, Neubert D et al (2016) Systemic inflammatory response after open, laparoscopic and robotic surgery in endometrial cancer patients. Anticancer Res 36:2909–2922
LeBlanc K, M. Sweitzer S, (2015) Systematic review of clinical evidence for local anesthetic wound infiltration in reduction of post-surgical pain. Internal Med Open Access. https://doi.org/10.4172/2165-8048.1000207
Martinschek A, Stumm L, Ritter M, Heinrich E, Bolenz C, Trojan L (2017) Prospective, controlled study of invasiveness and post-aggression metabolism in patients undergoing robotic-assisted radical prostatectomy. Urol Int 99:201–206. https://doi.org/10.1159/000478027
Pathak S, Goel N, Chowdhury I, Bhageria V (2016) Anaesthetic management of a patient with glucose-6-phosphate dehydrogenase deficiency undergoing robotic-assisted laparoscopic radical prostatectomy. J Soc Anesthesiol Nepal 3:93–95. https://doi.org/10.3126/jsan.v3i2.15621
Xu Q, Zhao B, Ye Y, Li Y, Zhang Y, Xiong X et al (2021) Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke. J Neuroinflammation 18:123. https://doi.org/10.1186/s12974-021-02137-8
Bailey EH, Glasgow SC (2015) Challenges in the medical and surgical management of chronic inflammatory bowel disease. Surg Clin North Am 95(1233–44):vii. https://doi.org/10.1016/j.suc.2015.08.003
Cohen BD, Marshall MB (2020) Robotic-assisted tracheobronchial surgery. J Thorac Dis 12:6173–6178. https://doi.org/10.21037/jtd.2020.03.05
Pelizzo G, Nakib G, Romano P, Avolio L, Mencherini S, Zambaiti E et al (2015) Five millimetre-instruments in paediatric robotic surgery: advantages and shortcomings. Minim Invasive Ther Allied Technol 24:148–153. https://doi.org/10.3109/13645706.2014.975135
Máca J, Peteja M (2007) Alarmins and surgical injury. Rozhl Chir 96:105–113
Spotnitz WD (2012) Getting to hemostasis: general and thoracic surgical challenges. Tex Heart Inst J 39:868–870
Muller R, Musikic P (1987) Hemorheology in surgery–a review. Angiology 38:581–592. https://doi.org/10.1177/000331978703800802
Wareing A (2017) Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Int J Nurs Pract. https://doi.org/10.1111/ijn.12552
Puca AA, Carrizzo A, Ferrario A, Villa F, Vecchione C (2012) Endothelial nitric oxide synthase, vascular integrity and human exceptional longevity. Immun Ageing 9:26. https://doi.org/10.1186/1742-4933-9-26
Pessoa RR, Maroni P, Kukreja J, Kim SP (2021) Comparative effectiveness of robotic and open radical prostatectomy. Transl Androl Urol 10:2158–2170. https://doi.org/10.21037/tau.2019.12.01
Wikiel KJ, Robinson TN, Jones EL (2021) Energy in robotic surgery. Ann Laparosc Endosc Surg. 6:9. https://doi.org/10.21037/ales.2020.03.06
Shakir N, Wong D, Cadeddu J, Roehrborn C (2019) Pd40–12 impact of single dose perioperative enoxaparin on rate of thromboembolic events following robotic assisted prostatectomy: a hospital system-wide analysis. J Urol. https://doi.org/10.1097/01.Ju.0000556544.63234.67
Waigankar SS, Yuvaraja TB, Dev P, Agarwal V, Pednekar AP, Kulkarni B (2021) Robotic Freyer’s prostatectomy: operative technique and single-center experience. Indian J Urol 37:247–253. https://doi.org/10.4103/iju.IJU_78_21
Pogatzki-Zahn E (2010) Prevention and therapy of prolonged, chronic pain after surgery. Anasth Intensivmed Notfallmed Schmerzther 45:496–503. https://doi.org/10.1055/s-0030-1262479. (Quiz 4)
Meghana N, Bharadwaj M, Goel N, Shukla S (2022) Endotracheal tube cuff pressure changes with pneumoperitoneum and steep head down position in patients undergoing robotic urogynecological surgeries—a prospective observational study. J Indian College Anaesthesiol. https://doi.org/10.4103/jica.jica_15_22
Faas CL, Acosta FJ, Campbell MD, O’Hagan CE, Newton SE, Zagalaniczny K (2002) The effects of spinal anesthesia vs epidural anesthesia on 3 potential postoperative complications: pain, urinary retention, and mobility following inguinal herniorrhaphy. AANA J 70:441–447
Watrowski R, Kostov S, Alkatout I (2021) Complications in laparoscopic and robotic-assisted surgery: definitions, classifications, incidence and risk factors - an up-to-date review. Wideochir Inne Tech Maloinwazyjne 16:501–525. https://doi.org/10.5114/wiitm.2021.108800
Saily VM, Petas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS (2014) Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 48:153–159. https://doi.org/10.3109/21681805.2013.817482
Naghi I, Seader K, Hashemi E, Sommers G, Anderson PS (2016) The role of robotic surgery versus laparotomy on the incidence of thromboembolism in uterine cancer [21P]. Obstet Gynecol 127:136S-S137. https://doi.org/10.1097/01.AOG.0000483550.73369.fc
Kapoor I, Rath G (2018) Robotized surgical assistant in neurosurgery: anaesthetic implications! J Neuroanaesthesiol Crit Care 03:151–152. https://doi.org/10.4103/2348-0548.182328
Zemmar A, Lozano AM, Nelson BJ (2020) The rise of robots in surgical environments during COVID-19. Nat Mach Intell 2:566–572. https://doi.org/10.1038/s42256-020-00238-2
Orsolya M, Daniela BS, Rodica R, Cipriana C, Attila MZ, Loan C (2012) Renal function monitoring in urogenital robot-assisted laparoscopic surgery performed in general anaesthesia. Med Con 23:13–18. https://doi.org/10.33311/medcon.2012.23.3.3
Martini A, Sfakianos JP, Paulucci DJ, Abaza R, Eun DD, Bhandari A et al (2019) Predicting acute kidney injury after robot-assisted partial nephrectomy: implications for patient selection and postoperative management. Urol Oncol 37:445–451. https://doi.org/10.1016/j.urolonc.2019.04.018
Bogdanov RR, Nurimanshin AF, Husaenova AA, Khasanov AR (2023) Anesthetic aspects of robot-assisted surgery (a review). Pacific Medical Journal. https://doi.org/10.34215/1609-1175-2023-1-11-18
Di Benedetto F, Petrowsky H, Magistri P, Halazun KJ (2020) Robotic liver resection: hurdles and beyond. Int J Surg 82S:155–162. https://doi.org/10.1016/j.ijsu.2020.05.070
Mori C, Iwasaki H, Sato I, Takahoko K, Inaba Y, Kawasaki Y et al (2023) Impact of intraoperative fluid restriction on renal outcomes in patients undergoing robotic-assisted laparoscopic prostatectomy. J Robot Surg. https://doi.org/10.1007/s11701-023-01610-1
Nomine-Criqui C, Demarquet L, Schweitzer ML, Klein M, Brunaud L, Bihain F (2020) Robotic adrenalectomy: when and how? Gland Surg 9:S166–S172. https://doi.org/10.21037/gs.2019.12.11
Ortenzi M, Ghiselli R, Baldarelli M, Cardinali L, Guerrieri M (2018) Is the bipolar vessel sealer device an effective tool in robotic surgery? A retrospective analysis of our experience and a meta-analysis of the literature about different robotic procedures by investigating operative data and post-operative course. Minim Invasive Ther Allied Technol 27:113–118. https://doi.org/10.1080/13645706.2017.1329212
Muaddi H, Hafid ME, Choi WJ, Lillie E, de Mestral C, Nathens A et al (2021) Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): a systematic overview of reviews. Ann Surg 273:467–473. https://doi.org/10.1097/SLA.0000000000003915
Lindfors A, Akesson A, Staf C, Sjoli P, Sundfeldt K, Dahm-Kahler P (2018) Robotic vs open surgery for endometrial cancer in elderly patients: surgical outcome, survival, and cost analysis. Int J Gynecol Cancer 28:692–699. https://doi.org/10.1097/IGC.0000000000001240
Yamamoto S (2020) Comparison of the perioperative outcomes of laparoscopic surgery, robotic surgery, open surgery, and transanal total mesorectal excision for rectal cancer: an overview of systematic reviews. Ann Gastroenterol Surg 4:628–634. https://doi.org/10.1002/ags3.12385
Khan H, Dhillon K, Mahapatra P, Popat R, Zakieh O, Kim WJ et al (2021) Blood loss and transfusion risk in robotic-assisted knee arthroplasty: a retrospective analysis. Int J Med Robot 17:e2308. https://doi.org/10.1002/rcs.2308
Salciccia S, Rosati D, Viscuso P, Canale V, Scarrone E, Frisenda M et al (2021) Influence of operative time and blood loss on surgical margins and functional outcomes for laparoscopic versus robotic-assisted radical prostatectomy: a prospective analysis. Cent European J Urol 74:503–515. https://doi.org/10.5173/ceju.2021.0177
Hockstein NG, Weinstein GS, O’Malley BW Jr (2005) Maintenance of hemostasis in transoral robotic surgery. ORL J Otorhinolaryngol Relat Spec 67:220–4. https://doi.org/10.1159/000088012
Becker F, Morgul H, Katou S, Juratli M, Holzen JP, Pascher A et al (2021) Robotic liver surgery—current standards and future perspectives. Z Gastroenterol 59:56–62. https://doi.org/10.1055/a-1329-3067
Ziegler S, Ortu A, Reale C, Proietti R, Mondello E, Tufano R et al (2008) Fibrinolysis or hypercoagulation during radical prostatectomy? An evaluation of thrombelastographic parameters and standard laboratory tests. Eur J Anaesthesiol 25:538–543. https://doi.org/10.1017/S0265021508003852
Ye L, Childers CP, de Virgilio M, Shenoy R, Mederos MA, Mak SS et al (2021) Clinical outcomes and cost of robotic ventral hernia repair: systematic review. BJS Open. https://doi.org/10.1093/bjsopen/zrab098
Fontalis A, Kayani B, Asokan A, Haddad IC, Tahmassebi J, Konan S et al (2022) Inflammatory response in robotic-arm-assisted versus conventional jig-based TKA and the correlation with early functional outcomes: results of a prospective randomized controlled trial. J Bone Joint Surg Am 104:1905–1914. https://doi.org/10.2106/JBJS.22.00167
Kayani B, Tahmassebi J, Ayuob A, Konan S, Oussedik S, Haddad FS (2021) A prospective randomized controlled trial comparing the systemic inflammatory response in conventional jig-based total knee arthroplasty versus robotic-arm assisted total knee arthroplasty. Bone Joint J 103-B:113–22. https://doi.org/10.1302/0301-620X.103B1.BJJ-2020-0602.R2
Colon KC, Seligsohn D, Salame G (2021) Transversus abdominis plane block in robotic gynecologic oncology surgery. Austin J Anesth Analg. https://doi.org/10.26420/austinjanesthesiaandanalgesia.2021.1097
Webster TM, Herrell SD, Chang SS, Cookson MS, Baumgartner RG, Anderson LW et al (2005) Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: a prospective assessment of postoperative pain. J Urol 174:912–4. https://doi.org/10.1097/01.ju.0000169455.25510.ff
Akdemir A, Yildirim N, Zeybek B, Karaman S, Sendag F (2015) Single incision trans-umbilical total hysterectomy: robotic or laparoscopic? Gynecol Obstet Invest 80:93–98. https://doi.org/10.1159/000370000
Tan YG, Allen JC, Tay KJ, Huang HH, Lee LS (2020) Benefits of robotic cystectomy compared with open cystectomy in an enhanced recovery after surgery program: a propensity-matched analysis. Int J Urol 27:783–788. https://doi.org/10.1111/iju.14300
Gul ZG, Katims AB, Winoker JS, Wiklund P, Waingankar N, Mehrazin R (2021) Robotic assisted radical cystectomy versus open radical cystectomy: a review of what we do and don’t know. Transl Androl Urol 10:2209–2215. https://doi.org/10.21037/tau.2019.11.32
Zhao H, Sun W (2022) Effect of enhanced recovery after surgery with integrated traditional chinese and western medicine on postoperative stress response of patients with gastrointestinal tumors. Comput Math Methods Med 2022:3663246. https://doi.org/10.1155/2022/3663246
Maerz DA, Beck LN, Sim AJ, Gainsburg DM (2017) Complications of robotic-assisted laparoscopic surgery distant from the surgical site. Br J Anaesth 118:492–503. https://doi.org/10.1093/bja/aex003
Campos J, Ueda K (2014) Update on anesthetic complications of robotic thoracic surgery. Minerva Anestesiol 80:83–88
El-Hakim A, Leung RA, Tewari A (2006) Robotic prostatectomy: a pooled analysis of published literature. Expert Rev Anticancer Ther 6:11–20. https://doi.org/10.1586/14737140.6.1.11
Zhang R, Li T, Ye L, Lin L, Wei Y (2022) The evidence behind robot-assisted abdominopelvic surgery. Ann Intern Med 175:W22. https://doi.org/10.7326/L21-0781
Oblak T (2021) The incidence of peripheral nerve injuries related to patient positioning during robotic assisted surgery: an evidence summary. J Perioper Nurs 34:49–53. https://doi.org/10.26550/2209-1092.1166
El Rassi I, El Rassi JM (2020) A review of haptic feedback in tele-operated robotic surgery. J Med Eng Technol 44:247–254. https://doi.org/10.1080/03091902.2020.1772391
Fotiou A, Iavazzo C (2022) Gynecologic robotic surgery: intraoperative complication and conversion rates. J Invest Surg 35:916–917. https://doi.org/10.1080/08941939.2021.1962440
Ackerman SJ, Daniel S, Baik R, Liu E, Mehendale S, Tackett S et al (2018) Comparison of complication and conversion rates between robotic-assisted and laparoscopic rectal resection for rectal cancer: which patients and providers could benefit most from robotic-assisted surgery? J Med Econ 21:254–261. https://doi.org/10.1080/13696998.2017.1396994
Gibber M, Lehr EJ, Kon ZN, Wehman PB, Griffith BP, Bonatti J (2014) Is there a role for robotic totally endoscopic coronary artery bypass in patients with a colostomy? Innovations (Phila) 9:448–450. https://doi.org/10.1177/155698451400900610
De la Harb Rosa A, Garcia-Castaneda J, Hsu CH, Zeng J, Batai K, Lee BR et al (2020) Perioperative outcomes of open vs. robotic radical cystectomy: a nationwide comparative analysis (2009-2014). Cent European J Urol. 73:427–31. https://doi.org/10.5173/ceju.2020.0230
Schlager JG, Hartmann D, Wallmichrath J, RuizSanJose V, Patzer K, French LE et al (2022) Patient-dependent risk factors for wound infection after skin surgery: a systematic review and meta-analysis. Int Wound J 19:1748–1757. https://doi.org/10.1111/iwj.13780
Simhal RK, Wang KR, Shah Y, Simon DP, Mark JR, Shah MS et al (2023) Risk analysis of open vs. robotic assisted radical cystectomy. J Clin Oncol 41:576. https://doi.org/10.1200/JCO.2023.41.6_suppl.576
Wang J, Johnson NW, Casey L, Carne PWG, Bell S, Chin M et al (2023) Robotic colon surgery in obese patients: a systematic review and meta-analysis. ANZ J Surg 93:35–41. https://doi.org/10.1111/ans.17749
Zarak A, Castillo A, Kichler K, de la Cruz L, Tamariz L, Kaza S (2015) Robotic versus laparoscopic surgery for colonic disease: a meta-analysis of postoperative variables. Surg Endosc 29:1341–1347. https://doi.org/10.1007/s00464-015-4197-7
Karategos A, Yassin N (2022) P557 Robotic surgery significantly improves outcomes for patients with inflammatory bowel disease. J Crohns Colitis 16:i502-i. https://doi.org/10.1093/ecco-jcc/jjab232.683
Ravendran K, Abiola E, Balagumar K, Raja AZ, Flaih M, Vaja SP et al (2023) A review of robotic surgery in colorectal surgery. Cureus 15:e37337. https://doi.org/10.7759/cureus.37337
Ito H, Moritake T, Isaka K (2022) Does the use of a uterine manipulator in robotic surgery for early-stage endometrial cancer affect oncological outcomes? Int J Med Robot 18:e2443. https://doi.org/10.1002/rcs.2443
Hori S, Nakai Y, Tomizawa M, Morizawa Y, Gotoh D, Miyake M et al (2022) Trends in primary treatment for localized prostate cancer according to the availability of treatment modalities and the impact of introducing robotic surgery. Int J Urol 29:1371–9. https://doi.org/10.1111/iju.15003
Gagnon LO, Goldenberg SL, Lynch K, Hurtado A, Gleave ME (2014) Comparison of open and robotic-assisted prostatectomy: the University of British Columbia experience. Can Urol Assoc J 8:92–7. https://doi.org/10.5489/cuaj.1707
Jimenez-Rodriguez RM, Flynn J, Patil S, Widmar M, Quezada-Diaz F, Lynn P et al (2021) Comparing outcomes of robotic versus open mesorectal excision for rectal cancer. BJS Open. https://doi.org/10.1093/bjsopen/zrab135
(2022) Scientific Impact Paper No. 71: Robotic surgery in gynaecology. The Obstetr Gynaecol. 24:298. https://doi.org/10.1111/tog.12842
Ornellas AA (2013) Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int 111:1010. https://doi.org/10.1111/j.1464-410X.2013.11733.x
Casarin J, Multinu F, Tortorella L, Cappuccio S, Weaver AL, Ghezzi F et al (2020) Sentinel lymph node biopsy for robotic-assisted endometrial cancer staging: further improvement of perioperative outcomes. Int J Gynecol Cancer 30:41–7. https://doi.org/10.1136/ijgc-2019-000672
Asanoma K, Yahata H, Okugawa K, Ohgami T, Yasunaga M, Kodama K et al (2022) Impact of obesity on robotic-assisted surgery in patients with stage IA endometrial cancer and a low risk of recurrence: an institutional study. J Obstet Gynaecol Res 48:3226–32. https://doi.org/10.1111/jog.15434
Martinez-Perez A, Carra MC, Brunetti F, de Angelis N (2017) Pathologic outcomes of laparoscopic vs open mesorectal excision for rectal cancer: a systematic review and meta-analysis. JAMA Surg 152:165665. https://doi.org/10.1001/jamasurg.2016.5665
Attaluri V, McLemore EC (2016) The cost of robotic surgery. Semin Colon Rectal Surg 27:134–5. https://doi.org/10.1053/j.scrs.2016.04.004
Oshimori N, Guo Y, Taniguchi S (2021) An emerging role for cellular crosstalk in the cancer stem cell niche. J Pathol 254:384–94. https://doi.org/10.1002/path.5655
Brzozowa M, Michalski M, Wyrobiec G, Piecuch A, Dittfeld A, Harabin-Slowinska M et al (2015) The role of Snail1 transcription factor in colorectal cancer progression and metastasis. Contemp Oncol (Pozn) 19:265–70. https://doi.org/10.5114/wo.2014.42173
Baldini E, Tuccilli C, Pironi D, Catania A, Tartaglia F, Di Matteo FM et al (2021) Expression and clinical utility of transcription factors involved in epithelial-mesenchymal transition during thyroid cancer progression. J Clin Med. https://doi.org/10.3390/jcm10184076
Niland S, Riscanevo AX, Eble JA (2021) Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int J Mol Sci. https://doi.org/10.3390/ijms23010146
Umamaheswari A, Katari S, Pasala C, Nalamolu R, Vankadoth U, Alexander S et al (2019) Pathophysiology of matrix metalloproteinases in breast cancer progression. J Clin Sci Res. https://doi.org/10.4103/jcsr.Jcsr_67_19
Sidorkiewicz I, Piskor B, Dabrowska E, Guzinska-Ustymowicz K, Pryczynicz A, Zbucka-Kretowska M et al (2019) Plasma levels and tissue expression of selected cytokines, metalloproteinases and tissue inhibitors in patients with cervical cancer. Anticancer Res 39:6403–12. https://doi.org/10.21873/anticanres.13854
Jin B, Zhang YY, Pan JX (2023) The role and significance of hepatic environmental cells in tumor metastatic colonization to liver. Sichuan Da Xue Xue Bao Yi Xue Ban 54:469–74. https://doi.org/10.12182/20230560301
Dahn ML, Marcato P (2020) Targeting the roots of recurrence: new strategies for eliminating therapy-resistant breast cancer stem cells. Cancers (Basel). https://doi.org/10.3390/cancers13010054
Colak S, Medema JP (2014) Cancer stem cells–important players in tumor therapy resistance. FEBS J 281:4779–91. https://doi.org/10.1111/febs.13023
Savelieva OE, Tashireva LA, Kaigorodova EV, Buzenkova AV, Mukhamedzhanov RK, Grigoryeva ES et al (2020) Heterogeneity of stemlike circulating tumor cells in invasive breast cancer. Int J Mol Sci. https://doi.org/10.3390/ijms21082780
Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS (2016) Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 60(3):289–334. https://doi.org/10.1111/aas.12651
Tang F, Tie Y, Lan TX, Yang JY, Hong WQ, Chen SY et al (2023) Surgical treatment of osteosarcoma induced distant pre-metastatic niche in lung to facilitate the colonization of circulating tumor cells. Adv Sci (Weinh). https://doi.org/10.1002/advs.202207518
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–70. https://doi.org/10.1126/science.1203486
Del Vecchio F, Martinez-Rodriguez V, Schukking M, Cocks A, Broseghini E, Fabbri M (2021) Professional killers: the role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J Extracell Vesicles 10:e12075. https://doi.org/10.1002/jev2.12075
Farhood B, Najafi M, Mortezaee K (2019) CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 234:8509–21. https://doi.org/10.1002/jcp.27782
Khan S, Jain M, Mathur V, Feroz SM (2016) Chronic inflammation and cancer: paradigm on tumor progression, metastasis and therapeutic intervention. Gulf J Oncolog 1:86–93
Do HTT, Lee CH, Cho J (2020) Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers (Basel). https://doi.org/10.3390/cancers12020287
Schmitt TM, Stromnes IM, Chapuis AG, Greenberg PD (2015) New strategies in engineering T-cell receptor gene-modified T cells to more effectively target malignancies. Clin Cancer Res 21:5191–7. https://doi.org/10.1158/1078-0432.CCR-15-0860
Pu Y, Ji Q (2022) Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front Immunol. https://doi.org/10.3389/fimmu.2022.874589
Kaptein P, Jacoberger-Foissac C, Dimitriadis P, Voabil P, de Bruijn M, Brokamp S et al (2022) Addition of interleukin-2 overcomes resistance to neoadjuvant CTLA4 and PD1 blockade in ex vivo patient tumors. Sci Transl Med 14:eabj9779. https://doi.org/10.1126/scitranslmed.abj9779
Gonzalez H, Hagerling C, Werb Z (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32:1267–84. https://doi.org/10.1101/gad.314617.118
Berraondo P, Minute L, Ajona D, Corrales L, Melero I, Pio R (2016) Innate immune mediators in cancer: between defense and resistance. Immunol Rev 274:290–306. https://doi.org/10.1111/imr.12464
Wang CS, Kozlow JR, Troost JP, Corcoran JF (2022) Reliability of a case-based oral examination by case and competency for evaluation of plastic surgery residents. Plastic Reconstr Surg Global Open. 10:8–9. https://doi.org/10.1097/01.Gox.0000834952.14979.78
Holoman M, Zahorec R, Kusy J, Pechan I (1995) Biochemical monitoring in patients during revascularization surgery. Vasa 24:23–8
Pechan I, Halcak L, Rendekova V, Barta E, Cornak V, Zahorec R et al (1993) Biochemical parameters in the blood of patients during open-heart surgery. I. Monitoring changes in energy metabolism. Bratisl Lek Listy 94:349–53
Rijs Z, Shifai AN, Bosma SE, Kuppen PJK, Vahrmeijer AL, Keereweer S et al (2021) Candidate biomarkers for specific intraoperative near-infrared imaging of soft tissue sarcomas: a systematic review. Cancers (Basel). https://doi.org/10.3390/cancers13030557
Jiang P (2018) Abstract B24: digital signatures of T cell dysfunction predict immunotherapy response. Cancer Immunol Res 6:B24. https://doi.org/10.1158/2326-6074.Tumimm17-b24
Algadi HH, Abou-Bakr AA, Jamali OM, Fathy LM (2020) Toluidine blue versus frozen section for assessment of mucosal tumor margins in oral squamous cell carcinoma. BMC Cancer 20:1147. https://doi.org/10.1186/s12885-020-07644-0
Schaefer JM, Barth CW, Davis SC, Gibbs SL (2019) Diagnostic performance of receptor-specific surgical specimen staining correlates with receptor expression level. J Biomed Opt 24:1–9. https://doi.org/10.1117/1.JBO.24.2.026002
Marochkov AV (2012) Control of laboratory parameters level as a component of anesthesia monitoring in patients undergoing abdominal surgery. Health and Ecology Issues. https://doi.org/10.51523/2708-6011.2012-9-3-18
Bay E, Dean-Ben XL, Pang GA, Douplik A, Razansky D (2015) Real-time monitoring of incision profile during laser surgery using shock wave detection. J Biophotonics 8:102–11. https://doi.org/10.1002/jbio.201300151
Ding J (2006) Realtime control algorithm for teleoperated surgery robot. Chin J Mech Eng. https://doi.org/10.3901/jme.2006.12.163
Andrew BY, Andrew EY, Cherry AD, Hauck JN, Nicoara A, Pieper CF et al (2018) Intraoperative renal resistive index as an acute kidney injury biomarker: development and validation of an automated analysis algorithm. J Cardiothorac Vasc Anesth 32:2203–9. https://doi.org/10.1053/j.jvca.2018.04.014
Matsubara TJ, Fujiu K, Shimizu Y, Oshima T, Matsuda J, Matsunaga H et al (2020) Fluoroless and contrast-free catheter ablation without a lead apron in routine clinical practice. Sci Rep 10:17096. https://doi.org/10.1038/s41598-020-74165-y
Connolly P (2004) The potential for biosensors in cardiac surgery. Perfusion 19:247–9. https://doi.org/10.1191/0267659104pf747oa
Ding D, Li J, Bai D, Fang H, Lin J, Zhang D (2020) Biosensor-based monitoring of the central metabolic pathway metabolites. Biosens Bioelectron 167:112456. https://doi.org/10.1016/j.bios.2020.112456
Perdomo SA, Marmolejo-Tejada JM, Jaramillo-Botero A (2021) Review—bio-nanosensors: fundamentals and recent applications. J Electrochem Soc. https://doi.org/10.1149/1945-7111/ac2972
Smith BR, Cheng Z, De A, Rosenberg J, Gambhir SS (2010) Dynamic visualization of RGD-quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small 6:2222–9. https://doi.org/10.1002/smll.201001022
Liu X, Braun GB, Zhong H, Hall DJ, Han W, Qin M et al (2016) Tumor-targeted multimodal optical imaging with versatile cadmium-free quantum dots. Adv Funct Mater 26:267–76. https://doi.org/10.1002/adfm.201503453
Bergholt MS, Lin K, Wang J, Zheng W, Xu H, Huang Q et al (2016) Simultaneous fingerprint and high-wavenumber fiber-optic Raman spectroscopy enhances real-time in vivo diagnosis of adenomatous polyps during colonoscopy. J Biophotonics 9:333–42. https://doi.org/10.1002/jbio.201400141
Khan H, Shah MR, Barek J, Malik MI (2023) Cancer biomarkers and their biosensors: a comprehensive review. TrAC Trends Anal Chem. https://doi.org/10.1016/j.trac.2022.116813
Ravalli A, Marrazza G (2015) Gold and magnetic nanoparticles-based electrochemical biosensors for cancer biomarker determination. J Nanosci Nanotechnol 15:3307–19. https://doi.org/10.1166/jnn.2015.10038
Lalvani SB (2019) Electrochemical biosensors for detection of cancer biomarkers. Int J Biosens Bioelectron. https://doi.org/10.15406/ijbsbe.2019.05.00170
Asuvaran A, Elatharasan G (2021) Design of two-dimensional photonic crystal based biosensors for abnormal tissues analysis. Silicon 14(12):1–8. https://doi.org/10.21203/rs.3.rs-628354/v1
Yin B, Wang X, Yuan F, Li Y, Lu P (2022) Research progress on the effect of gut and tumor microbiota on antitumor efficacy and adverse effects of chemotherapy drugs. Front Microbiol 13:899111. https://doi.org/10.3389/fmicb.2022.899111
Askari E, Seyfoori A, Amereh M, Gharaie SS, Ghazali HS, Ghazali ZS et al (2020) Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels. https://doi.org/10.3390/gels6020014
Hakim ML, Nahar N, Saha M, Islam MS, Reza HM, Sharker SM (2020) Local drug delivery from surgical thread for area-specific anesthesia. Biomed Phys Eng Express 6:015028. https://doi.org/10.1088/2057-1976/ab6a1e
Park CG, Hartl CA, Schmid D, Carmona EM, Kim HJ, Goldberg MS (2018) Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aar1916
Das KP (2022) Nanoparticles and convergence of artificial intelligence for targeted drug delivery for cancer therapy: current progress and challenges. Front Med Technol. 4:1067144. https://doi.org/10.3389/fmedt.2022.1067144
Bu LL, Yan J, Wang Z, Ruan H, Chen Q, Gunadhi V et al (2019) Advances in drug delivery for post-surgical cancer treatment. Biomaterials 219:119182. https://doi.org/10.1016/j.biomaterials.2019.04.027
Wolinsky JB, Colson YL, Grinstaff MW (2012) Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release 159:14–26. https://doi.org/10.1016/j.jconrel.2011.11.031
Yokoyama M (2005) Drug targeting with nano-sized carrier systems. J Artif Organs 8:77–84. https://doi.org/10.1007/s10047-005-0285-0
Dupoiron D, Douillard T, Carvajal G (2020) Usefulness of imaging for intrathecal drug delivery systems: an update. Med Res Arch. https://doi.org/10.18103/mra.v8i7.2175
Zerla PA, Canelli A, Cerne L, Caravella G, Gilardini A, De Luca G et al (2017) Evaluating safety, efficacy, and cost-effectiveness of PICC securement by subcutaneously anchored stabilization device. J Vasc Access 18:238–42. https://doi.org/10.5301/jva.5000655
Wang L, Wang H, Li HZ (2023) Real-time customized precision combination therapies based on potential actionability and coalterations to provide therapeutic opportunities for hyperprogressive disease after immune checkpoint inhibitor therapy. J Clin Oncol 41:14688. https://doi.org/10.1200/JCO.2023.41.16_suppl.e14688
Park SH, Kim KY, Kim YM, Hyung WJ (2023) Patient-specific virtual three-dimensional surgical navigation for gastric cancer surgery: a prospective study for preoperative planning and intraoperative guidance. Front Oncol 13:1140175. https://doi.org/10.3389/fonc.2023.1140175
Franklin WA, Carbone DP (2003) Molecular staging and pharmacogenomics. Clinical implications: from lab to patients and back. Lung Cancer 41(Suppl 1):147–54. https://doi.org/10.1016/s0169-5002(03)00158-2
Fuji L, Louw D (2007) Surgical robotics for patient safety in the perioperative environment: realizing the promise. Surg Innov 14:77–82. https://doi.org/10.1177/1553350607303880
Pilie PG, LoRusso PM, Yap TA (2017) Precision medicine: progress, pitfalls, and promises. Mol Cancer Ther 16:2641–4. https://doi.org/10.1158/1535-7163.MCT-17-0904
Mercogliano MF, Bruni S, Mauro FL, Schillaci R (2023) Emerging targeted therapies for HER2-positive breast cancer. Cancers (Basel). https://doi.org/10.3390/cancers15071987
Stukan AI, Goryainova AY, Riger NA, Sharov SV, Shatokhina AS, Chukhray OY et al (2021) Germinal <i>BRCA</i>-mutation significance in the tumor microenvironment formation Efficacy of PARP inhibition in late-line therapy of metastatic castration-resistant prostate cancer. Cancer Urol 17:85–94. https://doi.org/10.17650/1726-9776-2021-17-3-85-94
Bound NT, Vandenberg CJ, Kartikasari AER, Plebanski M, Scott CL (2022) Improving PARP inhibitor efficacy in high-grade serous ovarian carcinoma: a focus on the immune system. Front Genet 13:886170. https://doi.org/10.3389/fgene.2022.886170
Pacanowski M, Liu Q (2020) Precision medicine 2030. Clin Pharmacol Ther 107:62–4. https://doi.org/10.1002/cpt.1675
Roukos DH (2011) Cancer genome explosion and systems biology: impact on surgical oncology? Ann Surg Oncol 18:12–5. https://doi.org/10.1245/s10434-010-1355-y
Key BA, Mumba M (2019) Using precision medicine to individualize healthcare. Nursing 49:43–5. https://doi.org/10.1097/01.NURSE.0000569760.88666.61
Tobin AA, Little C, Schneider RJ, Thompson VM, Jannetto PJ, Wong SH et al (2008) Pharmacogenomic evaluation of a pediatric dextromethorphan fatality following cold medication therapy: the role of cytochrome P450 2D6 polymorphisms. The FASEB J. https://doi.org/10.1096/fasebj.22.1_supplement.1134.6
Matic M, de Hoogd S, de Wildt SN, Tibboel D, Knibbe CA, van Schaik RH (2020) OPRM1 and COMT polymorphisms: implications on postoperative acute, chronic and experimental pain after cardiac surgery. Pharmacogenomics 21:181–93. https://doi.org/10.2217/pgs-2019-0141
Silva CA, Ribeiro-Dos-Santos A, Goncalves WG, Pinto P, Pantoja RP, Vinasco-Sandoval T et al (2021) Can miRNA indicate risk of illness after continuous exposure to M. tuberculosis? Int J Mol Sci. https://doi.org/10.3390/ijms22073674
Deghmane AE, Taha MK (2021) Invasive bacterial infections in subjects with genetic and acquired susceptibility and impacts on recommendations for vaccination: a narrative review. Microorganisms. https://doi.org/10.3390/microorganisms9030467
Ercisli M, Lechun G, Azeez S, Hamasalih R, Song S, Aziziaram Z (2021) Relevance of genetic polymorphisms of the human cytochrome P450 3A4 in rivaroxaban-treated patients. Cell, Mol Biomed Rep 1:33–41. https://doi.org/10.55705/cmbr.2021.138880.1003
Chenoweth MJ, Giacomini KM, Pirmohamed M, Hill SL, van Schaik RHN, Schwab M et al (2020) Global pharmacogenomics within precision medicine: challenges and opportunities. Clin Pharmacol Ther 107:57–61. https://doi.org/10.1002/cpt.1664
Soares FFC, Ferreira D, Raimundini AA, Dionisio TJ, Dos Santos CF, Conti PCR et al (2023) Influence of genetic polymorphisms on mechanical pain sensitivity and endogenous pain modulation of trigeminal and spinal areas. J Oral Rehabil 50:39–53. https://doi.org/10.1111/joor.13384
Su H, Kwok KW, Cleary K, Iordachita I, Cavusoglu MC, Desai JP et al (2022) State of the art and future opportunities in mri-guided robot-assisted surgery and interventions. Proc IEEE Inst Electr Electron Eng 110:968–92. https://doi.org/10.1109/jproc.2022.3169146
Pellegrini G, Rasperini G, Pagni G, Giannobile WV, Milani S, Musto F et al (2017) Local wound healing biomarkers for real-time assessment of periodontal regeneration: pilot study. J Periodontal Res 52:388–96. https://doi.org/10.1111/jre.12403
Hall OM, Peer CJ, Figg WD (2018) Tissue preservation with mass spectroscopic analysis: implications for cancer diagnostics. Cancer Biol Ther 19:953–5. https://doi.org/10.1080/15384047.2018.1456610
Sikdar S, Ganguly K, Guha S, Bag S, Barman H (2020) Molecular imaging technology: a promising frontier of interventional radiology. SSRN Electron J. https://doi.org/10.2139/ssrn.3515891
Shurin MR, Baraldi JH, Shurin GV (2021) Neuroimmune regulation of surgery-associated metastases. Cells. https://doi.org/10.3390/cells10020454
Kranke P, Redel A, Schuster F, Muellenbach R, Eberhart LH (2008) Pharmacological interventions and concepts of fast-track perioperative medical care for enhanced recovery programs. Expert Opin Pharmacother 9:1541–64. https://doi.org/10.1517/14656566.9.9.1541
Haidegger T, Speidel S, Stoyanov D, Satava RM (2022) Robot-assisted minimally invasive surgery—surgical robotics in the data age. Proc IEEE 110:835–46. https://doi.org/10.1109/jproc.2022.3180350
Brodie A, Vasdev N (2018) The future of robotic surgery. Ann R Coll Surg Engl 100:4–13. https://doi.org/10.1308/rcsann.supp2.4
Black C, Voigts J, Agrawal U, Ladow M, Santoyo J, Moore C et al (2017) Open ephys electroencephalography (Open Ephys + EEG): a modular, low-cost, open-source solution to human neural recording. J Neural Eng 14:035002. https://doi.org/10.1088/1741-2552/aa651f
Funding
There was no financial support for this study.
Author information
Authors and Affiliations
Contributions
LM and FH: participated in article writing, AR: involved in article writing and final revision, FH and LM: contributed to drawing the manuscript tables and figures, and AN: consented to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human and animal participants
This article contains no studies with human participants or animals performed by the authors.
Consent for publication
This manuscript has been approved for publication by all the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mokhtari, L., Hosseinzadeh, F. & Nourazarian, A. Biochemical implications of robotic surgery: a new frontier in the operating room. J Robotic Surg 18, 91 (2024). https://doi.org/10.1007/s11701-024-01861-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11701-024-01861-6