Abstract
Traditional teaching suggests that prior pelvic operations, including prostatectomy, are a contraindication to laparoscopic inguinal hernia repair. Despite the growing use of robotic platforms in inguinal hernia repair, there are few studies describing robotic-assisted inguinal hernia repairs (RIHR) in this patient population. This study aims to demonstrate that RIHR is safe and effective in repairing inguinal hernias in patients who had previously undergone prostatectomy. We retrospectively reviewed RIHR cases performed from March 2017 to October 2021 by a single surgeon at our university-affiliated community hospital. Cases were reviewed for preoperative considerations, operative times and complications, and postoperative outcomes. A total of 30 patients with prior prostatectomy underwent transabdominal preperitoneal (TAPP) RIHR with mesh. Sixteen of the 30 patients had undergone robot-assisted laparoscopic prostatectomy (RALP), while 14 patients underwent open resection. Seven of the patients had received post-resection radiation and 12 had previous non-urologic abdominal operations. When compared to all RIHRs performed over the same period, duration of surgery was increased. There were no conversions to open surgery. Postoperatively, one patient developed a repair site seroma which resolved after 1 month. Mean follow-up time was 8.0 months. At follow-up, one patient reported experiencing intermittent non-debilitating pain at the repair site and one patient developed an inguinoscrotal abscess of unknown relation to the repair. No patients reported hernia recurrences nor mesh infection. This review suggests that TAPP RIHR can be a safe and effective approach to inguinal hernia repair in patients who have previously undergone prostatectomy, including those who received radiation and those who underwent either open or robotic resections.
Similar content being viewed by others
Introduction
Hernia repair is one of the most common operations performed in the US. Approximately, 75% of abdominal wall hernias are inguinal hernias with approximately 25% of men being affected in their lifetime [1]. For men who have undergone radical prostatectomy, the risk is as high as 38.7% [2, 3]. Historically, these men were not offered minimally invasive surgery for their hernia repairs as prior pelvic operations and radiation were considered contraindications to laparoscopic approaches [4]. However, there is evidence showing quicker recovery, less pain and earlier return to work with minimally invasive techniques [5]. Among these techniques, the transabdominal preperitoneal approach (TAPP) has the added advantage of examining the contralateral side for a defect and being able to repair bilateral inguinal hernias with the same incisions [6]. As awareness of these advantages grows, there is increasing interest in expanding patient access to minimally invasive hernia repair, even patients with prior contraindications such as pelvic surgery.
The emergence of robot-assisted surgery arguably has significant advantages in re-operative tissue planes with tools such as 3D visualization, wrist articulation, and improved surgeon ergonomics [7]. However, the evidence for robot-assisted laparoscopic inguinal hernia repair (RIHR) after prostatectomy is scarce with only one retrospective study reported to date [8]. In absence of strong evidence for RIHR or traditional laparoscopic hernia repair, current guidelines still recommend an open approach in these patients. Given the higher risk of inguinal hernia development following prostatectomy and the higher likelihood of recurrence after initial repair, there is need for greater research in this area [9]. The objective of this retrospective case series is to describe our experience with RIHR in this growing patient population and to share practical surgical techniques that can help the minimally invasive surgeon.
Methods
A retrospective chart review was performed after institutional review board (IRB) approval was obtained. All patients with a history of prostatectomy who underwent TAPP RIHR with mesh (ProGrip™ Laparoscopic Self-Fixating Mesh, Medtronic, Minneapolis, MN) by a single surgeon at a university-affiliated community hospital between March 2017 and October 2021 were identified. Demographic information (i.e., age and body mass index), prior pelvic and abdominal surgeries, and radiation history was reviewed for each patient. Intraoperative data including total operating times were analyzed along with need to convert to open repair and any intraoperative complications. Post-repair data were also gathered including pain, recurrences, and any postoperative complications.
Surgical technique
All surgeries were performed with use of a da Vinci Xi or Si Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). The mesh used was a self-fixating mesh designed for laparoscopic inguinal hernia repair (ProGrip™, Medtronic, Minneapolis, MN). The following is a general description of the TAPP surgical technique performed in these cases including specific considerations for this patient population.
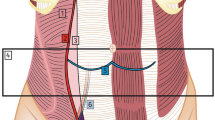
Preoperative Foley catheter is avoided to preserve any urethral strictures that may be protective for these patients in terms of maintaining urinary continence. Pneumoperitoneum is initially established with use of a Veress needle via supraumbilical transverse curvilinear incision. After establishing pneumoperitoneum to 12–15 mmHg, an 8 mm robotic port is placed in the supraumbilical midline. Two additional 8 mm ports are then placed approximately 8 cm lateral to the midline port on each the right and the left with each port positioned slightly more cephalad than the midline port. The patient is placed in a Trendelenburg position and the robot is docked with camera targeting the internal ring of the affected side for a unilateral hernia or in the pelvic midline for bilateral hernias. For instrumentation, a fenestrated bipolar grasper is used in the left hand and monopolar scissors are used in the right hand. For patients with a large hernia sac where reduction is challenging, a Caudiere grasper is exchanged for the scissors for the reduction. At the conclusion of the repair, a large needle-driver is used in the right hand for mesh positioning and re-approximation of the peritoneal flap.
The initial dissection begins with a transverse peritoneal incision 4 cm above the internal inguinal ring extending from the median umbilical ligament to the level of the anterior superior iliac spine. That peritoneal flap is then developed in the avascular plane down to the pelvic floor with the goal of exposing the testicular vascular bundle, the vas deferens, the psoas muscle, and the iliac artery. Interestingly, the vas deferens often appears normal even in patients who have undergone prostatectomy with vas deferens division. This suggests that the clipped vas deferens still provides one of the typical three sources of blood supply to the testicle (testicular artery, vas deferens via inferior vesicle artery, and small arterial branches from the inferior epigastric artery (cremasteric artery)). In post-prostatectomy patients, this aspect of the dissection is usually similar to patients without any prior pelvic surgery.
The medial dissection is usually more challenging in these patients, especially if the patient has received radiation treatments. Post-treatment tissue planes are distorted and firm. Tips to minimize risk of injury to surrounding structures include identifying the pubic tubercle early in the dissection, closely following that bone to the pubic symphysis, and being mindful of the iliac vein which can be retracted into a more medial position than normal. The most critical aspect of a TAPP dissection, in general, is the inferior medial dissection to ensure that the mesh in that location is not displaced by a distended bladder. This is the same location in which the iliac vein can become medialized. The data in the present study suggest that the surgeon has developed this space just large enough to accommodate circumferential coverage of the myopectineal orifice with a 10 × 15 cm piece of mesh but limits further dissection that would accommodate the placement of the preferred size of mesh (12 × 16 cm). The presenting surgeon favors the minimized risk of injury to surrounding structures over the benefit of larger mesh use in this patient population. Regardless of mesh size, assurance that the mesh lays flat with wide circumferential coverage of the myopectineal orifice along with assurance that the mesh does not roll or migrate with replacement of the peritoneal flap is key to minimize hernia recurrence. The conclusion of this repair occurs with peritoneal re-approximation with complete coverage of the mesh prosthetic. Scarring, especially with radiation treatment, can cause peritoneal fibrosis and contraction which limits the forgiveness of peritoneal stretching common in ‘normal’ TAPP repairs. Often the peritoneum will approximate without undue effort, but occasionally it will require patching with vicryl mesh with care taken to avoid any gaps or holes.
Results
During the study period, we identified 30 patients with prior prostatectomy who underwent TAPP RIHR. Patient characteristics and results are shown in Table 1, below. The mean age of the patients was 70 years (47–86) with a mean BMI of 27 (21–42.4). Sixteen of the 30 (53%) patients had undergone robot-assisted laparoscopic prostatectomy (RALP) while 14 (47%) patients underwent open resection. Seven (23%) of the patients had received post-resection radiation and 12 (40%) had previous non-urologic abdominal operations. When compared to all RIHRs performed over the same period, duration of surgery was increased. Total skin times for unilateral repairs after prostatectomy were 84.2 ± 20.1 min vs 74.2 ± 38.9 min for all unilateral RIHR’s performed by the same surgeon over the same period. Average skin time was even more prolonged for bilateral repairs, however, there were two outliers at 157 and 139 min and operative times for 2 out of the 13 bilateral repairs were not recorded. There were no conversions to open surgery. With a mean follow-up of 8.0 months (0.33–27.8), 2 patients had complications (repair site seroma and inguinoscrotal abscess), 1 patient complained of intermittent non-debilitating pain at the repair site, and no patients had recurrences of their hernia(s). The repair site seroma resolved without treatment after 1 month. The inguinoscrotal abscess was contralateral to the repair site and was found incidentally during hospitalization for a urinary tract infection. This occurred 3 weeks post-surgery and further evaluation revealed no evidence of immediate involvement of the pelvic floor nor within the preperitoneal plane in which the mesh rested. After treatment with antibiotics and local incision and drainage of the fluid collection from a scrotal approach, the patient recovered with no long-term morbidity.
Discussion
This study demonstrates that TAPP RIHR is both feasible and safe in patients with prior radical prostatectomy. No conversions to open repair were needed, no intraoperative complications occurred, and no recurrences were reported during follow-up. Minor complications, including the surgical site seroma and intermittent postoperative pain, were self-limiting. In the patient who developed an inguinoscrotal abscess, the RIHR surgery was unremarkable and his immediate postoperative period was notable only for asymptomatic, generalized soft tissue swelling in the area of repair with no overt fluid collection. While longer follow-up is needed to assess the true risk of recurrence in these patients, to date, there is no evidence of hernia recurrence nor mesh infection. Thus, it is unclear if the inguinoscrotal abscess was a complication of RIHR or an unrelated event. This 30-patient study is the largest such review to date and adds further support to the current literature showing that the TAPP approach is a good alternative for difficult hernia cases such as after radical prostatectomy.
Inguinal hernia is a known complication of radical prostatectomy. A meta-analysis by Zhu et al. found that inguinal hernia developed in 15.9% and 6.7% of patients who underwent either open or laparoscopic radical prostatectomy, respectively [10]. As our population ages, the number of patients with prior prostatectomy presenting with inguinal hernia is expected to increase. It is important to determine which surgical approaches can be safely and effectively performed in these patients with the lowest impact on quality of life.
Traditionally, prior pelvic surgery was considered a contraindication to laparoscopic hernia repair due to the resulting fibrosis that occurs in the preperitoneal space [11]. Since dissection of this space with subsequent mesh reinforcement is performed in laparoscopic techniques, anterior open repair was endorsed. However, minimally invasive techniques demonstrate benefits for patients undergoing inguinal hernia repair, including less scaring, decreased postoperative pain and quicker return to normal activity. As laparoscopic surgery has become more common over the past decade, there is a growing body of evidence that minimally invasive hernia repair is safe and effective after prior prostatectomy [9, 12,13,14,15]. Wauschkuhn et al. reported low morbidity and recurrence rates with TAPP inguinal hernia repair in 214 patients with prior prostatectomy and showed that with increasing surgeon experience, complications and hernia recurrence declined [12]. These results have been supported by subsequent studies with laparoscopic TAPP in patients with previous open and robot-assisted radical prostatectomy [8, 13, 16]. Thus, the evidence would suggest that patients with a history of prostatectomy can realize the same benefits of faster recovery and less pain after laparoscopic surgery as those without prior pelvic surgery [15].
The introduction of the robot into general surgery has increased the adoption of laparoscopic inguinal hernia repair. With a shorter learning curve than straight-stick laparoscopic techniques, use of the robotic platform for inguinal hernia repairs has grown more than 40-fold since 2012 [17]. However, relatively little literature exists regarding RIHR, especially in more complicated inguinal hernia cases such as those presented in the current study. In their recent report, Dewulf and colleagues were the first to report outcomes of TAPP RIHR after prostatectomy. In their cohort of 22 patients with prior transabdominal prostatectomy who underwent TAPP RIHR, the authors found the technique to be safe and feasible [8]. Our experience of 30 patients who underwent TAPP RIHR and who had prior open or laparoscopic prostatectomy supports the findings by Dewulf that robotic inguinal hernia repair is safe and effective in this patient population. We have provided useful technical suggestions that one can use to safely repair inguinal hernias in these patients. One of the biggest challenges of these re-operative hernia repairs is identifying landmarks after anatomical alterations of the abdominal wall and inguinal structures. From our experience, the robotic platform improves both the visualization and access to pelvic structures which may improve the outcomes of these complicated repairs. Successful hernia repair in these patients requires careful dissection of the prevesical space to avoid damage to the bladder. As noted by others, we have found that this space can become intensely scared after radical prostatectomy. The magnified, 3D visualization via the robotic platform enabled clear identification of specific tissue planes despite this. Robot-enabled wrist articulation facilitated relatively easy intracorporal mesh fixation and closure of the overlying peritoneum. From our experience with RIHR over the past 3 years, we expect the improved dexterity, precision and control of the robotic platform to broaden the applicability of the TAPP approach into these challenging inguinal hernias and change the standard of care.
Limitations
This study is limited due to its retrospective design, small sample size, and lack of head-to-head comparisons with other techniques. Therefore, no conclusions can be made regarding the best surgical approach for inguinal hernia repair in patients with prior prostatectomy. In addition, since our follow-up was relatively short, our reported outcomes may be limited to the operative and early postoperative courses. To evaluate recurrence rates in these patients after RIHR, longer follow-up period will be necessary. Thus, future large, randomized studies with longer follow-up periods should be conducted to compare different inguinal repair approaches in this patient population.
Conclusion
TAPP RIHR is a safe and effective approach to inguinal hernia repair in patients who have previously undergone prostatectomy, including those who received radiation and those who underwent either open or robotic prostate resections. Further higher-powered, multi-surgeon prospective studies are needed to validate these findings.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary material.
References
Hammoud M, Gerken J (2020) Inguinal Hernia. StatPearls, Treasure Island (FL)
Daniyal M et al (2014) Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac J Cancer Prev 15(22):9575–9578
Tao Li ZW (2013) The Diagnosis and Treatments of Inguinal Hernia after Radical Prostatectomy. Surg Sci 4:83–84
Carter J, Duh QY (2011) Laparoscopic repair of inguinal hernias. World J Surg 35(7):1519–1525
McCormack K et al (2003) Laparoscopic techniques versus open techniques for inguinal hernia repair. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001785
Memon MA, Fitzgibbons RJ Jr (1998) Assessing risks, costs, and benefits of laparoscopic hernia repair. Annu Rev Med 49:95–109
Donkor C et al (2017) Current perspectives in robotic hernia repair. Robot Surg 4:57–67
Dewulf M et al (2021) Robotic-assisted laparoscopic inguinal hernia repair after previous transabdominal prostatectomy. Surg Endosc. https://doi.org/10.1007/s00464-021-08497-9
Izadpanahi MH, Milasi R (2020) Evaluation of the results and complications of transabdominal preperitoneal laparoscopic inguinal hernia repair in patients with a history of radical prostatectomy. Urol J 17(1):24–29
Zhu S et al (2013) Risk factors and prevention of inguinal hernia after radical prostatectomy: a systematic review and meta-analysis. J Urol 189(3):884–890
Winslow ER, Quasebarth M, Brunt LM (2004) Perioperative outcomes and complications of open vs laparoscopic extraperitoneal inguinal hernia repair in a mature surgical practice. Surg Endosc 18(2):221–227
Wauschkuhn CA, Schwarz J, Bittner R (2009) Laparoscopic transperitoneal inguinal hernia repair (TAPP) after radical prostatectomy: is it safe? Results of prospectively collected data of more than 200 cases. Surg Endosc 23(5):973–977
Sakon M et al (2017) Laparoscopic inguinal hernioplasty after robot-assisted laparoscopic radical prostatectomy. Hernia 21(5):745–748
Ohuchi M et al (2019) Surgical technique and outcomes of transabdominal preperitoneal inguinal hernia repair after radical prostatectomy: dissection between the transversalis fascia and superficial layers of preperitoneal fascia. Hernia 23(1):167–174
Claus CM et al (2014) Laparoscopic inguinal hernioplasty after radical prostatectomy: is it safe? Prospect Clin Trial Hernia 18(2):255–259
Peitsch WKJ (2019) Laparoscopic transperitoneal inguinal hernioplasty (TAPP) after radical open retropubic prostatectomy: special features and clinical outcomes. Hernia 23(2):281–286
Sheetz KH, Claflin J, Dimick JB (2020) Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open 3(1):e1918911
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AR conceived this work; HF, AH, PC, and BL contributed to the acquisition of data; CL, SL, KV, HF and AR analyzed and interpreted the data; CL and KV wrote the manuscript; AR provided critical revision. All the authors were involved in the review and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of University of Oklahoma Health Sciences Center approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lade, C., Ford, H., Venincasa, K. et al. No prostate? No problem: robotic inguinal hernia repair after prostatectomy. J Robotic Surg 17, 1757–1761 (2023). https://doi.org/10.1007/s11701-023-01586-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-023-01586-y