Abstract
Spontaneous biliary-enteric fistula after laparoscopic cholecystectomy bile duct injury is an extremely rare entity. Y-en-Roux hepaticojejunostomy has been demonstrated to be an effective surgical technique to repair iatrogenic bile duct injuries. Seven consecutive patients underwent robotic-assisted (n = 5) and laparoscopic (n = 2) biliary-enteric fistula resection and bile duct repair at our hospital from January 2012 to May 2017. We reported our technique and described post-procedural outcomes. The mean age was 52.4 years, mostly females (n = 5). The mean operative time was 240 min for laparoscopic cases and 322 min for robotic surgery, and the mean estimated blood loss was 300 mL for laparoscopic and 204 mL for robotic cases. In both groups, oral feeding was resumed between day 2 or 3 and hospital length of stay was 4–8 days. Immediate postoperative outcomes were uneventful in all patients. With a median of 9 months of follow-up (3–52 months), no patients developed anastomosis-related complications. We observed in this series an adequate identification and dissection of the fistulous biliary-enteric tract, a safe closure of the fistulous orifice in the gastrointestinal tract and a successful bile duct repair, providing the benefits of minimally invasive surgery.
Similar content being viewed by others
Introduction
Class E is the most common major bile duct injury (BDI), manifesting as bile leak with peritonitis, biloma, external fistula, sepsis and secondary biliary cirrhosis [1, 2]. Despite the incidence of BDI in the laparoscopic cholecystectomy era and the large amount of information about its repair, very few papers have reported the finding of a biliary-enteric fistula as a manifestation of disease [2,3,4,5,6,7].
The incidence of all types of secondary biliary fistulae (internal and external) is low ranging from 0.3 to 0.6% of all cholecystectomies [1]. Biliary-enteric fistulae are primary the result of an inflammatory disease of the gallbladder (gallstones), and rarely the result of peptic ulcer, malignancy or trauma [8, 9].
The actual incidence of iatrogenic bile duct injuries is 0.8 per 1000 cholecystectomies [10], of which less than 1% leads to internal biliary fistula [5, 7], being choledocho-duodenal the most common type.
The aim of this study is to describe our minimally invasive approach to biliary-enteric fistula secondary to iatrogenic bile duct injury either with laparoscopic or robotic-assisted Y-en-Roux hepaticojejunostomy.
Materials and methods
A retrospective analysis of our prospectively collected database was performed between January 2012 and May 2017, during which patients with BDI secondary to cholecystectomy that presented with spontaneous biliary-enteric fistula were included. All patients required bile duct repair and were operated with laparoscopic or robotic-assisted surgery. Information regarding the demographics of patients, comorbidities, details of cholecystectomy, type of injury, preoperative and postoperative diagnostic work-up, therapeutic interventions, and postoperative results was recorded prospectively, with prior approval of the institutional research and ethical board of our hospital.
Preoperative patient evaluation
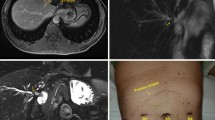
All patients are evaluated, stabilized and optimized for surgery by a multidisciplinary team including surgeons, nutritionists, infectologists, endoscopists and anesthesiologists. Antibiotic therapy is indicated as needed as well as correction of coagulopathy. All patients are studied preoperatively with tri-phasic computed tomography (CT) to rule out abscess, bile collections, or associated vascular injury. Definition of biliary anatomy and subsequent classification of the bile duct injury according to Strasberg [11] are made by preoperative magnetic resonance cholangiography (MRC) or endoscopic retrograde cholangiography (ERCP), see Fig. 1. All patients are diagnosed preoperatively and definition of the biliary-enteric fistula tract is achieved by MRC or ERCP.
Operative procedure
We approach all BDI with minimally invasive surgery, even if the index surgery was performed in an open fashion.
In all cases, we placed the patient in French position with the surgeon stood between the legs; the initial step is to perform a diagnostic laparoscopy. Following the inspection of the peritoneal cavity, bile collections are drained and intraabdominal adhesions are taken down.
The technique involves a laparoscopic Roux-en-Y loop construction. The jejunum is divided with endoscopic linear stapler and the mesentery with advanced energy devices. Then, the distal limb is placed close to the hilum in an antecolic position, ensuring adequate length (70 cm between the hepaticojejunostomy and the jejunojejunostomy) and no tension. Then, a side-to-side jejunojejunostomy is performed with endoscopic staplers and the enterotomies are closed with separate monofilament absorbable 2-0 sutures. After these steps are finished, we decided to procede either with laparoscopic surgery or with robotic-assisted surgery based on the availability of the equipment.
Laparoscopic approach
In all cases, peritoneal cavity is insufflated after a standard open Hasson or Veress needle technique through the transumbilical line and a 12-mm port is placed for a 30º laparoscopic lens. Three working ports are placed under direct vision in the following positions: 12-mm left para-median subcostal port, and 5-mm sub-xiphoid and right subcostal ports.
Robotic-assisted approach
Once the Roux-en-Y loop is finished, we proceeded to dock the robotic arms (Fig. 2). A 12-mm port is placed at the right para-umbilical area for the camera arm, 12-mm port in the left flank for robotic arm No. 1, and 8-mm port in right flank for robotic arm No. 2. One additional port is placed in the right side (between robotic arm No. 2 and camera arm) for assistant. The robotic system (Da Vinci Robotic Surgical System; Intuitive Surgical Inc., Sunnyvale, CA) is positioned at the head of the patient close to the left shoulder. We employed three arms: the robotic camera arm (for the binocular robotic scope) and two robotic working arms (we used grasper forceps, bipolar Maryland grasper, monopolar scissors and harmonic energy as needed). Laparoscopic assistant through the right flank is always needed for retraction, suction, insertion of sutures and retrieving of gauzes.
Common steps in laparoscopic and robotic-assisted approach
The inferior surface of the liver and porta hepatis is exposed in all cases. To assess liver status, biopsies are taken during this step. We proceeded with an intraoperative cholangiography (IOC) in order to define all segments of the intrahepatic biliary tree, identify possible intrahepatic stenosis and hepatolithiasis, and corroborate the class of the injury and define the fistulous tract.
In all cases, we took down the fistulous tract with blunt and sharp dissection. The bile duct remnant is fully inspected and ischemic or necrotic edges are excised until viable mucosa is found, then the hilar plate is lowered to obtain adequate length of the ducts. We identified the fistulous enteric orifice (pyloric, duodenal or colonic), then we resected an elliptical portion of the enteric wall in order to get well-vascularized mucosa and in all cases we performed primary closure of the defect with separate monofilament absorbable 2-0 sutures. These steps are successfully completed with the benefit of the magnified and high definition laparoscopic and three-dimensional robotic views.
In all cases, we performed cholangioscopy, through the sub-xiphoid port, in order to ensure adequate emptying and patency of intrahepatic biliary tree (Fig. 3). For this step, we employed a flexible gastroscope (Olympus GIF-H 180) [12].
The technique of repair consisted in a tension-free, side-to-side, mucosa-to-mucosa hepaticojejunostomy with an antecolic Roux-en-Y limb [13, 14]. In cases of E1 and E2 injuries, the common hepatic duct is opened longitudinally on its anterior surface and extended onto the anterior surface of the left hepatic duct or both the left and right hepatic ducts. For E3 injuries, a longitudinal ductotomy is performed in order to expose the confluence and part of the right hepatic duct [13, 14]. For E4 injuries, we built a neo-confluence, thus performing a single biliary-enteric anastomosis [14]. Patients with E4 injuries needed first a partial resection of segment IV to adequately expose the left duct [15].
Anastomosis between the jejunal limb and the previously dissected bile ducts is constructed with two running sutures of 4-0 monofilament absorbable barbed sutures (Stratafix®) in the case of robotic approach and 3-0 or 4-0 monofilament absorbable sutures with interrupted stitches and extracorporeal sliding knots [16] in the case of laparoscopic approach. The suture technique consisted in a standard side-to-side, single layer, anastomosis to the left hepatic duct/neo-confluence. Transhepatic stents are never employed.
We created an enteropexy of the blind segment of the jejunal biliary limb beneath sub-xiphoid region (access loop) [17]. This loop helps to gain future access to the hepaticojejunostomy and perform a percutaneous endoscopic rehabilitation in cases of stenosis of the anastomosis. A closed suction drain is placed routinely near the biliary-enteric anastomosis.
Postoperative management
Oral feeding is resumed once intestinal function is recovered. Patients are discharged home after fully tolerated normal oral diet and no need for intravenous drugs. The suction drain is removed before the patient is discharged. Patients are followed up through direct clinic encounters. Complications as well as mortality (divided as < 30 days from surgery and > 30 days after surgery) are recorded. Cholangitis, cholestasis or other event related to anastomotic failure are ruled out through clinical assessment and biochemical analysis (liver function tests). After patients achieved 1 year of follow-up, an MRC is performed to assess the anastomosis and proximal biliary three [17].
Results
A total of 7 patients referred to our hospital (between January 2012 and April 2017) with iatrogenic biliary duct injury presented with spontaneous biliary-enteric fistula. The mean age was 52.4 years, most patients were females (n = 5), and the mean BMI was 20.5 kg/m2. Patient’s baseline data are resumed in Table 1.
All biliary-enteric fistulae tract anatomy was defined preoperatively (with MRC or ERCP) and corroborated during surgical procedure (with IOC). The approach for the bile duct injury repair was laparoscopic (n = 2) or robotic (n = 5), based on the availability of the equipment.
Six patients presented without previous attempt to bile duct reconstruction and one patient with a previous attempt (choledocho–choledocho anastomosis with T-tube). The indications to operate in all patients were recurrent bouts of cholangitis and disturbing symptoms. The most frequent symptoms reported by the patients were pain (n = 7) and continuous bilious drainage (n = 5). Other symptoms reported by the patients were: nausea, vomiting, and pruritus. Patients with continuous bilious drainage had a preoperative external biliary fistula (n = 5).
Due to prolonged time of patient referral to our center, all repairs were classified as late (after 6 weeks of injury) [10].
Regarding intraoperative outcomes (Table 2), the mean operative time was 240 min for laparoscopic cases and 322 min for robotic surgery (including docking time); the mean estimated blood loss was 300 mL for laparoscopic cases and 204 mL for robotic cases. Of note, the technical difficulties encountered during surgeries were: the presence of dense adhesions, firm fibrosis around the fistulous tracts, and dissection until viable bile duct remnant mucosa appeared. In both groups, oral feeding was resumed between postoperative day 2 or 3, and mean hospital length of stay was 6.5 days (minimum–maximum: 4–8 days).
There were three complications within the first 30 days after surgery. Cases 1 and 6 required blood transfusion after the procedure. And case 3 developed a wound seroma. No re-admissions, re-operations or mortality were registered during the < 30 and > 30 day periods. With a median of 9 months of follow-up (minimum–maximum: 3–52 months), no patients developed anastomosis-related complications.
The histopathological changes found in liver biopsies included: portal inflammatory infiltrate, periductal fibrosis and/or intrahepatic cholestasis.
Discussion
To the best of the author’s knowledge, only 6 papers have been published reporting the presence of a biliary-enteric fistula secondary to iatrogenic bile duct injury and this paper is the first to describe the treatment with minimally invasive surgery (see Table 3) [2,3,4,5,6,7]. This very rarely entity should be early recognized due to the high risk of sepsis, cholangitis and liver parenchymal damage [5].
Pathophysiologic mechanism involved the formation of an internal biliary collection after BDI with subsequent erosion to the gastric or bowel wall [5]. Patients present with nausea, vomiting, chronic diarrhea, steatorrhea, right upper quadrant pain, peritonitis, pruritus, sepsis and cholestasis or they can be asymptomatic if the fistulous tract is patent [5,6,7]. For the evaluation of biliary-enteric fistula, computed tomography, magnetic resonance cholangiography, percutaneous transhepatic cholangiography and endoscopic retrograde cholangiography could be helpful in the identification of the fistulous tract [9].
Indications to treat biliary-enteric fistulas are: recurrent cholangitis episodes, associated external fistula, abscesses or biliary collections, stenosis of fistulous tract, and persisting symptoms [2, 7, 8, 18]. Surgical approach is based on the anatomy of the biliary tract and preoperative definition of the fistulous tract is important for planning the operation. All our patients presented either with recurrent cholangitis episodes, incapacitating symptomatology or external biliary drainage.
A non-operative approach is feasible if the fistula tract allows adequate biliary drainage and if the patient remains asymptomatic [7]. This requires long-term follow-up, and in some cases endoscopic rehabilitation with stent insertion in the fistula tract [1, 8].
If the patient requires surgery, following the approach of bile duct repair is indicated mainly with open surgery. A side-to-side Roux-en-Y hepaticojejunostomy is the best alternative in patients with complete section of the common bile duct, achieving complete rehabilitation in 75–98% of the patients in high volume centers [19]. The bile duct repair by minimally invasive surgery has been controversial, with a few cases reported around the globe; nevertheless, laparoscopic biliary-enteric derivations for other biliary and pancreatic pathologies have proved to be safe and feasible [17, 20].
The essential technical aspects for early and long-term success of BDI reconstruction are well-vascularized ducts, tension-free biliary epithelium-to-mucosa anastomosis with the largest possible diameter and complete drainage of all hepatic segments [11, 13, 21]; all these aspects can be achieved with both robotic-assisted and totally laparoscopic approach. If timely and adequate repair is undertaken, long-term results are excellent [7].
Minimal invasive surgery has well known and proven benefits such as reduction in intraoperative blood loss, postoperative pain, time to resume diet, hospital length of stay, surgical site infections, and cardiopulmonary complications, as well as the better quality of life and better cosmesis [12, 17]. In addition to these benefits, less pain is reported in robotic surgery due to minor trauma to the abdominal wall [22]. Also, in this new technical procedure (robotic assisted), a much better 3-D visualization and magnification is obtained and better ergonomics for the operating surgeon.
Our hospital is a specialized minimally invasive surgical center to which cases of complex BDI from other centers are referred. We begin our experience in laparoscopic bile duct repairs and later on we move to robotic-assisted repairs [12, 17]. Although this is a technical report with a small case series, we believed that results are promising, providing the patients the benefits of laparoscopic/robotic surgery while preserving the surgical principles of open bile duct repairs.
Conclusion
Spontaneous biliary-enteric fistula after laparoscopic cholecystectomy bile duct injury is an extremely rare entity. We observed in this series an adequate identification and dissection of the fistulous biliary-enteric tract, a safe closure of the fistulous orifice in the gastrointestinal tract and a successful bile duct repair, providing all the benefits of minimally invasive surgery. Although good outcomes are achievable, the minimally invasive treatment of complex biliary pathologies requires advanced laparoscopic, robotic and endoscopic skills, as well as experience in biliary-enteric reconstructions.
References
Crespi M, Montecamozzo G, Foschi D (2016) Diagnosis and treatment of biliary fistulas in the laparoscopic era. Gastroenterol Res Pract. https://doi.org/10.1155/2016/6293538
Yilmaz S, Akici M, Okur N, Türel S, Ers O (2016) Spontaneous postoperative choledochoduodenal fistula due to bileduct injury following laparoscopic cholecystectomy. Int J Surg Case Rep 25:199–202
Hunt DR, Blumgart LH (1980) Iatrogenic choledochoduodenal fistula: an unsuspected cause of postcholecystectomy symptoms. Br J Surg 67:10–13
Asbun HJ, Rossi RL, Lowell JA, Munson JL (1993) Bile duct injury during laparoscopic cholecystectomy: mechanism of injury, prevention, and management. World J Surg 17:547–552
Munene G, Graham JA, Holt RW, Johnson LB, Marshall HP (2006) Biliary-colonic fistula: a case report and literature review. Am Surg 72:347–350
Özogul YB, Özer I, Orug T, Ulas M, Ercan M, Parlak E, Bostanci B, Seven C, Atalay F, Akoglu M (2009) Spontaneous hepaticoduodenal fistula functioning like a bilioenteric anastomosis following bile duct injury: case report. Turk J Gastroenterol 20(3):220–223
Macedo FIB, Casillas VJ, Davis JS, Levi JU, Sleeman D (2013) Biliary-colonic fistula caused by cholecystectomy bile duct injury. Hepatobiliary Pancreat Dis Int 12:443–445
Maier J, Rudez J, Huber A (2008) Two possibly iatrogenic biliary–duodenal fistulas in a single patient after medical and surgical interventions. J Can Chir 51:28–29
Hong T, Xu X, He X, Qu Q, Li B, Zheng C (2014) Choledochoduodenal fistula caused by migration of endoclip after laparoscopic cholecystectomy. World J Gastroenterol 20(16):4827–4829
Dominguez-Rosado I, Sanford DE, Liu J, Hawkins WG, Mercado MA (2016) Timing of surgical repair after bile duct injury impacts postoperative complications but not anastomotic patency. Ann Surg 264:544–553
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180(1):101–125
Cuendis-Velazquez A, Trejo-Avila ME, Rosales-Castañeda E, Cárdenas-Lailson E, Rojano-Rodríguez ME, Romero-Loera S, Sanjuan-Martínez CA, Moreno-Portillo M (2017) Laparoscopic choledochoduodenostomy. Cir Esp 95(7):397–402
Winslow ER, Fialkowski EA, Linehan DC, Hawkins WG, Picus DD, Strasberg SM (2009) “Sideways’’: result of repair of biliary injuries using a policy of side-to-side hepatico-jejunostomy. Ann Surg 249(3):426–434
Mercado MA, Chan C, Orozco H, Tielve M, Hinojosa CA (2003) Acute bile duct injury. The need for a high repair. Surg Endosc 9:1351–1355
Mercado MA, Orozco H, de la Garza L, Lopez-Martinez LM, Contreras A, Guillen-Navarro E (1999) Biliary duct injury: partial segment IV resection for intrahepatic reconstruction of biliary lesions. Arch Surg 134(9):1008–1010
Pereira-Graterol FA, Moreno-Portillo M (2004) A New technique for tying the Gea extracorporeal knot for endoscopic surgery. J Laparoendosc Adv Surg Tech 14(6):403–406
Cuendis-Velázquez A, Morales-Chávez C, Aguirre-Olmedo I, Torres-Ruiz F, Rojano-Rodríguez M, Fernández-Álvarez L, Cárdenas-Lailson E, Moreno-Portillo M (2016) Laparoscopic hepaticojejunostomy after bile duct injury. Surg Endosc 30(3):876–882
Costi R, Random B, Violi V, Scatton O, Sarli L, Soubrane O, Dousset B, Montariol T (2009) Cholecystocolonic fistula: facts and myths. A review of the 231 published cases. J Hepatobiliary Pancreat Surg 16:8–18
Mercado MA, Franssen B, Domínguez I, Arriola-Cabrera JC, Ramírez-del Val F, Elnecavé-Olaiz A, Arámburo-Garcia R, García A (2011) Transition from a low-to a high-volume center bile duct repair: changes in technique and improved outcome. HPB (Oxford) 13(11):767–773
Giulianotti PC, Quadri P, Durgam S, Bianco FM (2017) Reconstruction/repair of iatrogenic biliary injuries is the robot offering a new option? Short clinical report. Ann Surg. https://doi.org/10.1097/SLA.0000000000002343
Stewart L, Way L (2009) Laparoscopic bile duct injuries: timing of surgical repair does not influence success rate. A multivariate analysis of factors influencing surgical outcomes. HPB 11:516–522
Prasad A, De S, Mishra P, Tiwari A (2015) Robotic assisted Roux-en-Y hepaticojejunostomy in a post-cholecystectomy type E2 bile duct injury. World J Gastroenterol 21(6):1703–1706
Funding
No external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Cuendis-Velázquez, Trejo-Ávila, Rodríguez-Parra, Bada-Yllán, Morales-Chávez, Fernández-Álvarez, Cárdenas-Lailson, Romero-Loera, Rojano-Rodríguez and Moreno-Portillo declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cuendis-Velázquez, A., Trejo-Ávila, M.E., Rodríguez-Parra, A. et al. Minimally invasive approach (robotic and laparoscopic) to biliary-enteric fistula secondary to cholecystectomy bile duct injury. J Robotic Surg 12, 509–515 (2018). https://doi.org/10.1007/s11701-017-0774-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-017-0774-1