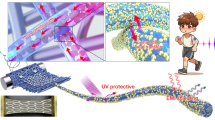
Abstract
UV-readable fluorescent ink formulations find versatile applications in various fields including information encryption, automated identification systems, security markers and optical devices. In this context, a new bithiophene-based chalcone (BTCF) that exhibits good solution phase and solid-state fluorescence was synthesized as a colourant for formulating an eco-friendly UV fluorescent ink. The molecule demonstrated good thermal stability and photophysical features including intramolecular charge transfer, confirmed through emission studies in THF–hexane mixtures with varying hexane content. The intense greenish yellow solid-state fluorescence emission displayed by BTCF was exploited by using it as a colourant in a water-based fluorescent ink formulation. Further, the ink was used to print a fast-drying solid patch on an UV dull paper substrate using flexography technique. The analysis of colorimetric, densitometric and rub resistance properties of the printed paper samples demonstrated good fluorescence, moderate photostability and good rub resistance, and hence could be used for security printing applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescent inks have extensive applications in wide areas including security printing, smart packaging products, certificates, information storage, bioimaging, highlighting markers, fluorescent tags, nanoelectronics, etc. (Liu et al. 2019). Moreover, there is a high demand for water-based fluorescent ink formulations, which is a greener approach wherein the water replaces the organic solvents (Muthamma et al. 2020). They are more environment-friendly as they emit less volatile organic compounds, thereby lesser pollution and better safety as the main benefits. In addition, these inks being less flammable aids in easier storage when compared to solvent-based inks. These eco-friendly fluorescent inks are popular for flexography printing, as they can be easily dried on highly absorbent substrates with excellent permeability, and can also withstand high temperature (Ramirez and Tumolva 2018; Jingxiang et al. 2019; Zołek-Tryznowska and Izdebska 2013; Zołek-tryznowska et al. 2015). Security printing using fluorescent inks can be adopted to realize various types of anticounterfeit technologies such as water marking, barcode, smart packaging and information encryption (Abdelhameed et al. 2021). Therefore, the use of luminescent pigments with good optical properties suitable for security printing have gained wide research interest.
The pigments used in fluorescent security inks should satisfy few stringent requirements including small particle size suitable to all printing processes, excellent resistance to commonly used solvents, chemicals, acids, soaps and detergents, along with good optical properties, lightfastness and rub resistance. Lanthanide-doped molecules (Yao et al. 2019), carbon dots (Qu et al. 2012; Kalytchuk et al. 2018), plasmonic materials (Tian et al. 2016; Duempelmann et al. 2017) and conventional organic materials have been investigated as pigments in security ink formulations in the recent past. Conventional organic molecules that own different carbon frameworks and/or carry other heteroatoms (N, O, S, etc.) including coumarin (Ataeefard and Nourmohammadian 2015; Talebnia et al. 2020), fluoran (Yang et al. 2019), benzothiazole (Echeverri et al. 2020), imidazole (Nadamani et al. 2019), tetraphenylethylene (Peng et al. 2019), naphthalene (Chen et al. 2019b), anthracene (Prusti and Chakravarty 2019) and pyrene (Wakchaure et al. 2019) have been investigated as pigments in solvent-based fluorescent inks. However, only few organic molecules such as coumarin (Ataeefard and Nourmohammadian 2015), fluorene (Muthamma et al. 2021) and pyrene (Chen et al. 2019a; Bhagya et al. 2021) have been explored as colourants in water-based security inks.
Bithiophene is a naturally occurring environmentally stable heterocycle with two coplanar rings, each incorporating a sulphur atom. Formerly, the use of this moiety in optical applications was limited due to low fluorescence quantum yields in both solid and solution state. The efficient intersystem crossing of heavy sulphur atom, well-organized solid-state packing, relaxation of excited state through interannular rotations within the molecular framework and aggregation-induced quenching might have contributed to the low emission quantum yield of thiophene-based materials (Rasmussen et al. 2015). Regardless of this limitation, various tactics have been developed to fabricate highly fluorescent thiophene-based materials over the last few years. Recent advances in constructing highly fluorescent materials by smartly tuning the inherent bithiophene-based structural framework offer ample impetus to construct newer derivatives for emissive applications. These sulphur containing small molecules are of substantial interest for versatile applications in organic electronics, as fluorescent biomarkers, sensors, security agents, etc. due to the possibility of controlled tuning of absorption and emission colour and good charge mobility (Perepichka and Perepichka 2009). Nevertheless, this molecular framework has not been investigated till date as a colourant in water-based flexo-ink formulations.
Chalcones are widely used for various applications due to their excellent fluorescent properties (Komarova et al. 2015), mainly due to the push–pull conjugation effect. Moreover, these materials have good thermal stability which is essential for long life time when used in various applications such as for printing or in electronic devices (Karuppusamy et al. 2017). Hence, the present study focuses on the synthesis as well as structural, optical, and thermal characterization of BTCF, a 2,2′-bithiophene incorporated chalcone with delocalized π-electrons and push–pull conjugation. Further, BTCF was used as a fluorescent pigment to formulate a water-based, eco-friendly ink for flexography printing. The fast-drying prints which displayed good optical and rub resistance features could be used for wide range of applications.
Results and discussion
Structural characterization of BTCF
The base catalysed aldol condensation between 2,2′-bithiophene-5-carboxaldehyde and 4-fluoroacetophenone generated the chalcone 3-([2,2′-bithiophen]-5-yl)-1-(4-fluorophenyl)prop-2-en-1-one (BTCF). Scheme 1 depicts the synthetic pathway for the chalcone.
The chemical structure of BTCF was established using spectral techniques as given below. The IR spectrum established the presence of enone linkage in the molecule (Fig. S1). The NMR spectra: 1H (Fig. S2a), 19F (Fig. S3) and 13C (Fig. S4) were in conformity with the chemical structure of the new chalcone. The Fig. S2b depicts the 1H NMR spectrum of the solvent used. All the eleven protons in BTCF resonated between 6.9 and 8.0 ppm. The carbonyl carbon resonated at 187.95 ppm in the 13C spectra, whereas the 19F spectra provided the evidence of fluorine in the structural framework of BTCF. The mass spectrum (Fig. S5) was in agreement with the molecular mass of the bithiophene derived chalcone.
3-([2,2'-bithiophen]-4-yl)-1-(4-fluorophenyl)prop-2-en-1-one (BTCF): golden yellow (84.75%) m.p:116–118 °C; FTIR (cm−1): 1645 (C=O str.), 1562 (C=C str.), 1018 (C–F str.); 1H NMR (400 MHz, CDCl3, ppm): δ 6.985–7.006 (t, 1H), 7.089–7.132 (m, 3H), 7.167–7.241 (m, 4H), 7.816–7.854 (d, 1H), 7.965–7.999 (dd, 2H); 19F NMR (376 MHz, CDCl3, ppm): δ–105.69; 13C NMR (100 MHz, CDCl3, ppm): δ 115.65, 115.87, 119.73, 124.64, 124.97, 125.91, 128.19, 130.92, 131.01, 133.69, 134.52, 136.68, 137.24, 138.85, 140.93, 166.88, 187.95; Mass spectra C17H11FOS2 (m/z): 315.0155.
Optical features and thermal stability of BTCF
The absorption and emission spectral traces of the chalcone were analysed to comprehend the optical properties. As presented in Fig. S6, the solution of BTCF in THF (1 × 10−5 M) showed two distinguished absorption bands at ~ 273 nm and ~ 398 nm due to the electronic transitions of the conjugated structural framework (Wang et al. 2017a, b; Zhang et al. 2017; Yang et al. 2020). The intense solid-state emission of BTCF is observed at 550 nm as shown in Fig. 1a, and the respective chromaticity diagram (Fig. 1b) indicates greenish yellow-coloured fluorescence. The emission spectra of BTCF solution in THF (1 × 10−5 M) depicted in Fig. 1c exhibits two prominent peaks at around ~ 408 nm and 432 nm when excited at 370 nm. Further, the emission features of BTCF in 1 × 10−5 M THF/THF–hexane mixtures were examined to check the effect of polarity of solvents on the fluorescence emission. The spectral variations of BTCF perceived in pure THF and upon increasing percentage volume of non-polar hexane is shown in Fig. 1d. The emission was blue-shifted from 432 to 425 nm with decrease in intensity, indicating the presence of an intramolecular charge transfer (ICT) process in the molecule (Sowmiya et al. 2011).
Thermal stability of pigments can be determined by thermogravimetric analysis (TGA), and the thermogram of BTCF as shown in Fig. S7 displayed a two-step weight loss. The chalcone is stable up to 250 °C as evident from the TGA plot and an initial 87% weight loss is detected from ~ 253 to 361 °C, which might be due to the partial decomposition of the compound (Naik et al. 2020).
Assessment of fluorescence property of the printed paper samples
As fluorescent materials have been widely used for various applications, especially security printing, BTCF was used as a pigment to formulate a fluorescent ink, which showed bright greenish yellow fluorescence when exposed to 365 nm UV light (Fig. S8a). The ink formulation, which was prepared as discussed in the experimental section comprised of BTCF pigment that served as the colouring and fluorescing component in the ink, whereas the vehicle of the ink consisted mainly of water as solvent along with other additives (Muthamma et al. 2021). The ink formulation with particle size of ~ 5 µm was used to print a patch on UV dull paper substrate using flexography. The printed paper samples dried quickly at room temperature and exhibited a greenish yellow fluorescence emission under 365 nm UV light source as portrayed in Fig. S8b. Further the excitation and the emission spectra of the printed sample (BP1) as shown in Fig. 2a were analysed. Upon excitation at 475 nm, the prints emitted at 540 nm. A slight blue-shift in the emission of the prints compared to the solid-state emission of BTCF (550 nm) could be due to the presence of ink components. The ink was further manually coated onto the UV dull paper using a zero numbered bar coater and the photographs of the coated paper (BC1) in daylight and under 365 nm UV light is presented in Fig. S8c. The intensity of emission from the coated paper (BC1) is found to be more than that of the print (BP1) as evident from Fig. 2b because of a thicker ink layer deposited during the coating process. Furthermore, the photostability of the prints were investigated by passing the printed paper sample under 300 W UV light for 0 (BP-0), 10 (BP-10), 20 (BP-20), 30 (BP-30), 40 (BP-40) and 50 (BP-50) passes, wherein the samples are exposed to UV light for 3–4 s during each cycle. The emission spectra of these samples are shown in Fig. 2c and it is perceived that fluorescence intensity of the UV exposed samples decreased with increase in exposure cycles.
a Excitation and emission spectra of the printed paper sample (BP1); \(\lambda_{{{\text{ex}}}}\) = 475 nm, b emission spectra of the unprinted (blank), printed (BP1) and coated (BC1) paper samples, and c emission spectra of printed paper samples after different cycles of exposure to 300 W UV light (30 m/min)
Colorimetric analysis of the printed paper samples
The colorimetric measurements are easy and simple and can be used to determine the colour coordinates. In general, the L* a* b* international standard colour space specifies the numerical colour values as it is closer to the human perception of colour. The colour space and the values are utilized to develop and visualize colour in 2D or 3D spaces (Subhashree et al. 2017). The colour transformations of the printed samples were investigated and analysed using CIELAB colour space measurements. The L* a* b* colour coordinates of the flexo-printed paper samples for different cycles of UV exposure are listed in Supplementary Table S1, and the respective colorimetric plots are depicted in Fig. 3a. The unprinted paper sample was slightly off-white with 94.5 (L*), − 0.2 (a*), 3.9 (b*), 0.6 (ΔE) and 0.1 (density) as the colorimetric values. Upon increasing the number of UV exposure cycles, the L* a* b* values were found to slightly deviate from the unprinted sample with a shade alteration from greenish yellow to pale yellow as observed from Fig. 3. The ΔE and densitometric plots are depicted in Fig. 3b and 3c.
The rub resistance of the print is yet another important parameter that determines the degree of repeated rubbing or scuffing the print can withstand. The rub test was performed to stimulate the amount of damage on repeated rubbing and to assess the ability of the print to resist it. The rub test as described in the experimental section was performed, where the printed samples were tested for 25, 50, 75 and 100 rubs, and after each set of rubs, the colorimetric values were recorded. The values obtained are presented in Table S1 and pictorially depicted in Fig. 3d, which indicate that there is negligible change in the colour parameters between the rubbed and the zero-rubbed samples. Moreover, the good rub resistance feature of the print is also evident from the ΔE and density values, which did not alter significantly as displayed in the plots (Fig. 3b and 3c).
Surface morphological topographies of the print
SEM and AFM techniques were used to examine the surface topographical features of the unprinted and printed samples, and the resulting microscopic images are presented in Fig. 4. The unprinted substrate exhibited a fibrous surface texture in the SEM images (Fig. 4a), whereas the printed paper sample posed a smoother surface (Fig. 4b) because the flexo-print creates a uniform and consistent ink film on the porous and fibrous paper substrate. Furthermore, from the AFM images displayed in Fig. 4c and d, the maximum roughness value (Rmax) for the printed paper sample was 146 nm when compared to 346 nm of the unprinted paper sample (Table S2). It is evident that the ink film has decreased the surface roughness of the paper.
Conclusion
In summary, we have synthesized a bithiophene-based chalcone (BTCF) for its plausible use as a pigment in UV-readable fluorescent ink formulation, which can extend real-life applications. The chemical structure of the colourant was established using spectral techniques. The fluorescent chalcone displayed several appealing properties including ICT and good thermal stability, and therefore served as a viable functional colourant for formulating a water-based eco-friendly ink. Flexography technique was used to effectively print the environment-friendly ink onto the porous paper surfaces. The prints demonstrated good adhesion and fast-drying on the porous substrate, moderate photostability, good abrasion resistance and fluorescence indicating its potential application in various fields including information encryption, data security, automatic identification, anticounterfeiting and optical devices (Nair et al. 2019).
Experimental
The chemicals and solvents used for the synthesis of BTCF were procured from Sigma-Aldrich and Spectrochem Chemicals Pvt. Ltd.
Synthesis of 3-([2,2′-bithiophen]-5-yl)-1-(4-fluorophenyl)prop-2-en-1-one (BTCF)
About 10% NaOH solution (0.5 mL) was added dropwise to a mixture of 4-fluoroacetophenone (1 mmol) and 2,2′-bithiophene-5-carboxaldehyde (1 mmol) in ethanol and stirred at room temperature (RT) for 5 h. The precipitate of BTCF formed was filtered, washed with water and dried.
The melting point of BTCF was determined using open capillary method. The FTIR and NMR spectra were recorded in ATR Shimadzu-IR Spirit analyser and 400 MHz Bruker spectrometer using CDCl3 as solvent, respectively. The Xevo QToF MS system (Waters, USA) was used to record the mass spectrum (HR-LCMS) of BTCF. The absorption and emission studies were performed using 1800 Shimadzu UV–visible spectrophotometer and JASCO spectrofluorometer FP 8300, respectively. The TGA was performed under nitrogen atmosphere at 10 °C min−1 as the heating rate using HITACHI Simultaneous Thermogravimetric Analyzer STA7000 series.
The components used for preparing the ink formulation including acrylic emulsions, solvents and additives were obtained from Kamson’s Pvt. Ltd, DOW Chemical Company, BASF—Chemicals and Evonik Industries. A mixture (wt%) of BTCF (12.36), acrylic emulsion 1 (66.24), wetting and dispersing agent (1.80) and diethylene glycol monoethyl ether (carbitol-19.60) were milled in the Llyod’s pigment muller 92 N at 40 Kgf to obtain the ink concentrate. The remaining components were dispersed in the ink concentrate to obtain the final ink composition (wt%): pigment BTCF (7.70), acrylic emulsion 1 (52.50), acrylic emulsion 2 (15.05), wax (1.70), de-foamer (1.25), wetting and dispersing agent (1.15), carbitol (12.20), butyl carbitol (2.00), water (4.90) and 25% ammonia solution (1.55). Zehnter grindometer was used to measure the particle size of the ink.
The UV dull paper was used as substrate for printing, which was obtained from Manipal Press, Pvt Ltd, Manipal, India. The flexography printing was carried out using a RK Flexiproof 100 apparatus at a speed of 75 m/min with 9.5 BCM anilox roll, photopolymer plate and a chambered doctor blade inking system. The photostability of the ink was studied using a Hari Impex LAB UV 83002 instrument by exposing the prints under UV light of 300 W and belt rotation speed of 30 m/min. The rub test was carried out using a Sutherland ink rub tester by placing the printed paper against an unprinted paper on the test rubber pad with 4 lbs for 100 rubs. The colorimetric data were recorded using Xrite I1 Pro spectrophotometer. The CIE 3D colour coordinates explored includes L* (indicates lightness; a value of 0 and 100 represent black and white, respectively), a* (green for negative and red for positive a* values) and b* (blue for negative and yellow for positive b* values), where the a* and b* values can range from − 120 to 120. ΔE gives the colour differences and is calculated using the equation below (Zołek-Tryznowska and Izdebska 2013; Muthamma et al. 2021).
where \(\left( {{\Delta }L^{*} } \right)\) = L*(u)–L*, \(\left( {{\Delta }a^{*} } \right)\) = a*(u)–a* and \(\left( {{\Delta }b} \right)\) = b*(u)–b* are the differences between the printed paper samples and (u) is the unprinted sample/blank paper sample (Zołek-tryznowska et al. 2015).
The surface morphologies of the printed and unprinted paper samples were recorded using Carl Zeiss EVO 18 analytical scanning electron microscope (SEM) and INNOVA SPM atomic force microscope (AFM).
Data availability
The data are available upon reasonable request from the first author (KM).
References
Abdelhameed MM, Attia YA, Abdelrahman MS, Khattab TA (2021) Photochromic and fluorescent ink using photoluminescent strontium aluminate pigment and screen printing towards anticounterfeiting documents. Lumin 36:865–874. https://doi.org/10.1002/bio.3987
Ataeefard M, Nourmohammadian F (2015) Producing fluorescent digital printing ink: investigating the effect of type and amount of coumarin derivative dyes on the quality of ink. J Lumin 167:254–260. https://doi.org/10.1016/j.jlumin.2015.06.042
Bhagya RS, Sunil D, Shetty P, Kagatikar S, Wagle S, Melroy Lewis P, Kulkarni SD, Kekuda D (2021) Water-based flexographic ink using chalcones exhibiting aggregation-induced enhanced emission for anti-counterfeit applications. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.117974
Chen L, Hu B, Zhang J, Zhang J, Huang S, Ren P, Li H (2019a) A facile synthesis of 1, 3, 6, 8-pyrenesulfonic acid tetrasodium salt as a hydrosoluble fluorescent ink for anti-counterfeiting applications. RSC Adv 9(1):476–481
Chen S, Zhang W, Jia Q, Meng Y, Wang K, Hu Z (2019b) Dimethylamino naphthalene-based cyanostyrene derivatives with stimuli responsive luminescent properties. Dye Pigment. https://doi.org/10.1016/j.dyepig.2019.107700
Duempelmann L, Müller JA, Lütolf F, Gallinet B, Ferrini R, Novotny L (2017) Controlling the color of plasmonic substrates with inkjet printing. Adv Opt Mater 5:1700153–1700157. https://doi.org/10.1002/adom.201700153
Echeverri M, Ruiz C, Gámez-valenzuela S, Navarro MA, Gutiérrez-puebla E, Serrano JL, Delgado MCR, Gómez-lor B (2020) A stimuli-responsive benzothiadiazole derivative as a dopant for rewritable polymer blends. ACS Appl Mater Interfaces 12:10929–10937. https://doi.org/10.1021/acsami.9b21209
Jingxiang X, Jinyao L, Haichao L, Mingming Z, Jifei C (2019) Research progress on water-based ink drying technology. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/565/1/012017
Kalytchuk S, Wang Y, Poláková K, Zbořil R (2018) Carbon dot fluorescence-lifetime-encoded anti-counterfeiting. ACS Appl Mater Interfaces 10:29902–29908. https://doi.org/10.1021/acsami.8b11663
Karuppusamy A, Vandana T, Kannan P (2017) Pyrene based chalcone materials as solid state luminogens with aggregation-induced enhanced emission properties. J Photochem Photobiol A Chem 345:11–20. https://doi.org/10.1016/j.jphotochem.2017.05.026
Komarova KG, Sakipov SN, Plotnikov VG, Alfimov MV (2015) Luminescent properties of chalcone and its aminoderivatives. J Lumin 164:57–63. https://doi.org/10.1016/j.jlumin.2015.03.021
Liu Y, Han F, Li F, Zhao Y, Chen M, Xu Z, Zheng X, Hu H, Yao J, Guo T, Lin W, Zheng Y, You B, Liu P, Li Y, Qian L (2019) Inkjet-printed unclonable quantum dot fluorescent anti-counterfeiting labels with artificial intelligence authentication. Nat Commun 10:2409. https://doi.org/10.1038/s41467-019-10406-7
Muthamma K, Sunil D, Shetty P (2020) Luminophoric organic molecules for anticounterfeit printing ink applications: an up-to-date review. Mater Today Chem. https://doi.org/10.1016/j.mtchem.2020.100361
Muthamma K, Sunil D, Shetty P, Kulkarni SD, Anand PJ, Kekuda D (2021) Eco-friendly flexographic ink from fluorene-based Schiff base pigment for anti-counterfeiting and printed electronics applications. Prog Org Coat 161:106463. https://doi.org/10.1016/j.porgcoat.2021.106463
Nadamani MP, Mahmoodi NO, Mamaghani M (2019) Photochromic properties of novel one-pot multicomponent synthesized tetraarylimidazoles. ChemistrySelect. https://doi.org/10.1002/slct.201901755
Naik VS, Patil PS, Wong QA, Quah CK, Gummagol NB, Jayanna HS (2020) Molecular structure, linear optical, second and third-order nonlinear optical properties of two non-centrosymmetric thiophene-chalcone derivatives. J Mol Struct 1222:128901. https://doi.org/10.1016/j.molstruc.2020.128901
Nair KS, Abhilash P, Surendran KP (2019) Silica-based organic-inorganic hybrid fluorescent ink for security applications. ACS Omega 4:2577–2583. https://doi.org/10.1021/acsomega.8b03313
Peng S, Wen J, Hai M, Yang Z, Yuan X, Wang D, Cao H, He W (2019) Synthesis and application of reversible fluorescent photochromic molecules based on tetraphenylethylene and photochromic groups. New J Chem. https://doi.org/10.1039/c8nj05388j
Perepichka IF, Perepichka DF (2009) Handbook of thiophene-based materials: applications in organic electronics and photonics. Wiley
Prusti B, Chakravarty M (2019) Carbazole-anthranyl π-conjugates as small and stable aggregation-induced emission-active fluorogens: serving as a reusable and efficient platform for anticounterfeiting applications with an acid key and multicolor ink for a quill pen. ACS Omega 4:16963–16971. https://doi.org/10.1021/acsomega.9b02277
Qu S, Wang X, Lu Q, Liu X, Wang L (2012) A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew Chemie Int Ed 51:12215–12218. https://doi.org/10.1002/anie.201206791
Ramirez JCC, Tumolva TP (2018) Analysis and optimization of water-based printing ink formulations for polyethylene films. Appl Adhes Sci 6:1–21. https://doi.org/10.1186/s40563-017-0102-z
Rasmussen SC, Evenson SJ, McCausland CB (2015) Fluorescent thiophene-based materials and their outlook for emissive applications. Chem Commun 51:4528–4543. https://doi.org/10.1039/c4cc09206f
Sowmiya M, Tiwari AK, Sonu SSK (2011) Study on intramolecular charge transfer fluorescence properties of trans-4-[4′-(N,N′-dimethylamino)styryl]pyridine: effect of solvent and pH. J Photochem Photobiol A Chem 218:76–86. https://doi.org/10.1016/j.jphotochem.2010.12.006
Subhashree SN, Sunoj S, Xue J, Bora GC (2017) Quantification of browning in apples using colour and textural features by image analysis. Food Qual Saf 1:221–226. https://doi.org/10.1093/fqsafe/fyx021
Talebnia F, Nourmohammadian F, Bastani S (2020) Development of novel fluorescent offset ink based on coumarin dyes: synthesis and properties. Prog Org Coat 77:1351–1359. https://doi.org/10.1016/j.porgcoat.2014.04.022
Tian L, Liu KK, Fei M, Tadepalli S, Cao S, Geldmeier JA, Tsukruk VV, Singamaneni S (2016) Plasmonic nanogels for unclonable optical tagging. ACS Appl Mater Interfaces 8:4031–4041. https://doi.org/10.1021/acsami.5b11399
Wakchaure VC, Das T, Santhosh S (2019) Boron-conjugated pyrenes as fluorescence-based molecular probes and security markers. ChemPlusChem. https://doi.org/10.1002/cplu.201900280
Wang H, Sun C, Chen X, Zhang Y, Colvin VL, Rice Q, Seo J, Feng S, Wang S, Yu WW (2017a) Excitation wavelength independent visible color emission of carbon dots. Nanoscale 9:1909–1915. https://doi.org/10.1039/c6nr09200d
Wang S, Cheng Z, Song X, Yan X, Ye K, Liu Y, Yang G, Wang Y (2017b) Highly efficient long-wavelength thermally activated delayed fluorescence oleds based on dicyanopyrazino phenanthrene derivatives highly efficient long-wavelength thermally activated delayed fluorescence oleds based on dicyanopyrazino phenanthrene derivat. ACS Appl Mater Interfaces 9:9892–9901. https://doi.org/10.1021/acsami.6b14796
Yang J, Ho Y, Chan Y (2019) Ultrabright fluorescent polymer dots with thermochromic characteristics for full-color security marking. ACS Appl Mater Interfaces 11:29341–29349. https://doi.org/10.1021/acsami.9b10393
Yang XC, Li Q, Tang M, Yang YL, Yang W, Hu JF, Pu XL, Liu J, Zhao JT, Zhang ZJ (2020) One stone, two birds: pH-and temperature-sensitive nitrogen-doped carbon dots for multiple anticounterfeiting and multiple cell imaging. ACS Appl Mater Interfaces 12:20849–20858. https://doi.org/10.1021/acsami.0c02206
Yao W, Tian Q, Wu W (2019) Tunable emissions of upconversion fluorescence for security applications. Adv Opt Mater 7:1–19. https://doi.org/10.1002/adom.201801171
Zhang Z, Chang H, Xue B, Han Q, Lü X, Zhang S, Li X, Zhu X, Wong WK, Li K (2017) New transparent flexible nanopaper as ultraviolet filter based on red emissive Eu(III) nanofibrillated cellulose. Opt Mater (Amst) 73:747–753. https://doi.org/10.1016/j.optmat.2017.09.039
Zołek-tryznowska Z, Izdebska J, Tryznowski M (2015) Branched polyglycerols as performance additives for water-based flexographic printing inks. Prog Org Coat 78:334–339. https://doi.org/10.1016/j.porgcoat.2014.07.015
Zołek-Tryznowska Z, Izdebska J (2013) Flexographic printing ink modified with hyperbranched polymers: Boltorn™ P500 and Boltorn™ P1000. Dye Pigment 96:602–608. https://doi.org/10.1016/j.dyepig.2012.10.003
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muthamma, K., Gouda, B.M., Sunil, D. et al. Water-based fluorescent flexo-ink for security applications. Chem. Pap. 77, 4033–4040 (2023). https://doi.org/10.1007/s11696-023-02765-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02765-9