Abstract
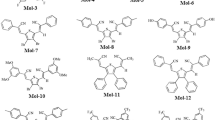
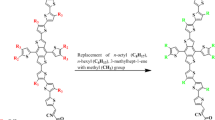
Arylated-p-benzoquinone derivatives have been designed for strong optoelectronic properties by employing density functional theory (DFT) and time-dependent density functional theory (TD-DFT). In this study, excitation energies, geometries of the ground state and excited state, dipole moment, and absorption spectra have been assessed using different DFT functionals like B3LYP, CAM-B3LYP, and ωB97XD with 6–31/G (d, p) basis set for broad understanding about structural properties at different functionals. CAM-B3LYP functional showed high resemblance with experimentally reported benzoquinone reference molecule. This study successfully predicted that designed compounds exhibits strong optoelectronic characteristics and provides an active tool for further designing and synthesize the strong UV active compounds with benzoquinone core unit.




Similar content being viewed by others
Availability of data
The data should be provided upon reasonable request.
Code availability
This research work has been carried out with Gauss View 5.0 software.
References
Ali U, Ans M, Iqbal J, Iqbal MA, Shoaib M (2019a) Benchmark study of benzamide derivatives and four novel theoretically designed (L1, L2, L3, and L4) ligands and evaluation of their biological properties by DFT approaches. J Mol Model 25(8):1–9. https://doi.org/10.1007/s00894-019-4115-3
Ali U, Javed A, Ramzan H, Shoaib M, Raza A, Khalil MT, Cheng S-B, Iqbal J (2020) Molecular designing of naphthalene diimide based fullerene-free small organic solar cell-Acceptors with high photovoltaic performance by density functional theory. Spectrochim Acta A Mol Biomol Spectrosc 228:117685. https://doi.org/10.1016/j.saa.2019.117685
Ali U, Javed A, Shoaib M, Kashif M, Raza A, Cheng S-B, Iqbal J (2019b) Designing difluoro substituted benzene ring based fullerene free acceptors for small Naphthalene Di-Imide based molecules with DFT approaches. Opt Quantum Electron 51(10):1–23. https://doi.org/10.1007/s11082-019-2047-x
Ali U, Javed A, Tallat A, Iqbal J, Raza A (2019c) Molecular designing of four high performance pyrazine-based non-fullerene acceptor materials with naphthalene diimide-based small organic solar cells. J Mol Model 25(2):1–12. https://doi.org/10.1007/s00894-019-3932-8
Abraham I, Joshi R, Pardasani P, Pardasani R (2011) Recent advances in 1, 4-benzoquinone chemistry. J Braz Chem Soc 22(3):385–421. https://doi.org/10.1590/S0103-50532011000300002
Ali U, Tariq A, Kiran A, Abbas F, Khalil MT (2021) Tuning the absorption and optoelectronic properties of naphthalene diimide based solar cells with non-fullerene acceptors. Chem Pap. https://doi.org/10.1007/s11696-021-01671-2
Abbas M, Ali U, Faizan M, Siddique MBA (2021) Spirofluorene based small molecules as an alternative to traditional non-fullerene acceptors for organic solar cells. Opt Quant Electron 53(5):1–14. https://doi.org/10.1007/s11082-020-02672-3
Ajmal M, Ali U, Javed A, Tariq A, Arif Z, Iqbal J, Shoaib M, Ahmed T (2019) Designing indaceno thiophene–based three new molecules containing non-fullerene acceptors as strong electron withdrawing groups with DFT approaches. J Mol Model 25(10):1–9. https://doi.org/10.1007/s00894-019-4198-x
Bringmann G, Mutanyatta-Comar J, Knauer M, Abegaz BM (2008) Knipholone and related 4-phenylanthraquinones: structurally, pharmacologically, and biosynthetically remarkable natural products. Nat Prod Rep 25(4):696–718. https://doi.org/10.1039/B803784C
Carrasco IJ, Marquez MC, Xue Y, Ma Y, Cowan DA, Jones BE, Grant WD, Ventosa A (2008) Sediminibacillus halophilus gen. nov., sp. nov., a moderately halophilic, Gram-positive bacterium from a hypersaline lake. International Int J Syst Evol Microbiol 58(8):1961–1967. https://doi.org/10.1099/ijs.0.65790-0
El-Najjar N, Gali-Muhtasib H, Ketola RA, Vuorela P, Urtti A, Vuorela H (2011) The chemical and biological activities of quinones: overview and implications in analytical detection. Phytochem Rev 10(3):353. https://doi.org/10.1007/s11101-011-9209-1
Frisch, M., Trucks, G., Schlegel, H. B., Scuseria, G., Robb, M., Cheeseman, J., . . . Petersson, G. (2009). Gaussian 09, revision a. 02, gaussian. Inc., Wallingford, CT, 200.
George JH, Baldwin JE, Adlington RM (2010) Enantiospecific, biosynthetically inspired formal total synthesis of (+)-liphagal. Org Lett 12(10):2394–2397. https://doi.org/10.1021/ol100756z
Guido CA, Knecht S, Kongsted J, Mennucci B (2013) Benchmarking time-dependent density functional theory for excited state geometries of organic molecules in gas-phase and in solution. J Chem Theory Comput 9(5):2209–2220. https://doi.org/10.1021/ct400021c
Hasegawa T, Mochida T, Kondo R, Kagoshima S, Iwasa Y, Akutagawa T, Nakamura T, Saito G (2000) Mixed-stack organic charge-transfer complexes with intercolumnar networks. Phys Rev B 62(15):10059. https://doi.org/10.1103/PhysRevB.62.10059
Hassan Z, Ullah I, Ali I, Khera RA, Knepper I, Ali A, Patonay T, Villinger A, Langer P (2013) Synthesis of tetraaryl-p-benzoquinones and 2, 3-diaryl-1, 4-naphthoquinones via Suzuki-Miyaura cross-coupling reactions. Tetrahedron 69(2):460–469. https://doi.org/10.1016/j.tet.2012.11.040
Hussain Z, Shoaib M, Ali U, Ramzan H, Ali M, Tariq A, Naqash M (2020) Theoretical study of isoxazoles and their derivatives for evaluating its pharmaceutical properties with density functional theory. J Comput Chem Mol Model 4(3):415–426. https://doi.org/10.25177/JCCMM.4.3.RA.10623
Iftikhar T, Ali U, Shoaib M (2020) Theoretical study of α, β unsaturated carbonyl thiophene derivatives to investigate optoelectronic properties toward organic photovoltaics. J Mol Model 26(12):1–8. https://doi.org/10.1007/s00894-020-04597-w
Koyama J (2006) Anti-infective quinone derivatives of recent patents. Recent Pat Anti-Infect Drug Discovery 1(1):113–125. https://doi.org/10.2174/157489106775244073
Latouche C, Palazzetti F, Skouteris D, Barone V (2014) High-accuracy vibrational computations for transition-metal complexes including anharmonic corrections: ferrocene, ruthenocene, and osmocene as test cases. J Chem Theory Comput 10(10):4565–4573. https://doi.org/10.1021/ct5006246
Mallakin A, Dixon DG, Greenberg BM (2000) Pathway of anthracene modification under simulated solar radiation. Chemosphere 40(12):1435–1441. https://doi.org/10.1016/S0045-6535(99)00331-8
Promkatkaew M, Suramitr S, Karpkird T, Ehara M, Hannongbua S (2013) Absorption and emission properties of various substituted cinnamic acids and cinnamates, based on TDDFT investigation. Int J Quantum Chem 113(4):542–554. https://doi.org/10.1002/qua.24169
Rouf SA, Mares J, Vaara J (2015) 1H chemical shifts in paramagnetic Co (II) pyrazolylborate complexes: A first-principles study. J Chem Theory Comput 11(4):1683–1691. https://doi.org/10.1021/acs.jctc.5b00193
Smith DG, Patkowski K (2015) Benchmarking the CO2 adsorption energy on carbon nanotubes. J Phys Chem C 119(9):4934–4948. https://doi.org/10.1021/jp512926n
Yu H-Z, Fu F, Zhang L, Fu Y, Dang Z-M, Shi J (2014) Accurate predictions of C-SO 2 R bond dissociation enthalpies using density functional theory methods. Phys Chem Chem Phys 16(38):20964–20970. https://doi.org/10.1039/C4CP02005G
Zhao Y, Yihuan L, Li R, He J, Zhang H, Wang X, Ge Z, Li R (2018) Discovery and optimization of 2-thio-5-amino substituted benzoquinones as potent anticancer agents. Eur J Med Chem 149:1–9. https://doi.org/10.1016/j.ejmech.2018.02.059
Zhu X-Q, Wang C-H (2010) Accurate estimation of the one-electron reduction potentials of various substituted quinones in DMSO and CH3CN. J Org Chem 75(15):5037–5047. https://doi.org/10.1021/jo100735s
Acknowledgements
The authors acknowledge the resources provided for this computational study to Punjab Bio-Energy Institute (PBI), Jhang road, Faisalabad and Chemistry department, University of Agriculture, Faisalabad, Pakistan.
Funding
No funding was received to carry out this research work.
Author information
Authors and Affiliations
Contributions
The authors Adeela Kiran and Usman Ali are equally contributed for this research work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest for disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiran, A., Ali, U., Hussain, A. et al. Molecular designing of tetra-aryl-p-benzoquinones derivatives toward strong optical properties. Chem. Pap. 75, 6661–6671 (2021). https://doi.org/10.1007/s11696-021-01834-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01834-1