Abstract
Purpose
Understanding the effects of Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) on adipose tissue physiology is important for the treatment of obesity-related metabolic disorders. By using robust mouse models of bariatric surgery that closely resemble those performed in humans, we can compare the effects of RYGB and VSG on adipose physiology in the absence of post-operative confounds such as diet and lifestyle changes.
Materials and Methods
RYGB and VSG were compared using a diet-induced mouse model of obesity. High-fat diet (HFD) was administered post-operatively and changes to white and brown adipose tissue were evaluated, along with alterations to weight, glucose homeostasis, dyslipidemia, and insulin sensitivity.
Results
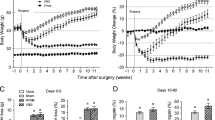
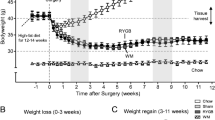
After prolonged exposure to high-fat diet post-operatively, RYGB was effective in achieving sustained weight loss, while VSG unexpectedly accelerated weight gain rates. The resolution of obesity-related comorbidities such as glucose and insulin intolerance, dyslipidemia, and insulin sensitivity was improved after RYGB, but not for VSG. In RYGB, there were improvements to the function and health of white adipose tissue, enhanced brown adipose metabolism, and the browning of subcutaneous white adipose tissue, with no comparable changes seen for these factors after VSG. Some markers of systemic inflammation improved after both RYGB and VSG.
Conclusion
There are significantly different effects between RYGB and VSG when HFD is administered post-operatively and robust mouse models of bariatric surgery are used. RYGB resulted in lasting physiological and metabolic changes but VSG showed little difference from that of its sham-operated, DIO counterpart.
Graphical abstract









Similar content being viewed by others
References
Bayham BE, Greenway FL, Bellanger DE, et al. Early resolution of type 2 diabetes seen after Roux-en-Y gastric bypass and vertical sleeve gastrectomy. Diabetes Technol Ther. 2012;14(1):30–4.
Lupoli R, Lembo E, Saldalamacchia G, et al. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464–74.
McCracken E, Wood GC, Prichard W, et al. Severe anemia after Roux-en-Y gastric bypass: a cause for concern. Surg Obes Relat Dis. 2018;14(7):902–9.
Benaiges D, Goday A, Ramon JM, et al. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis. 2011;7(5):575–80.
Colquitt JL et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641.
Nosso G et al. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res. 2016;48(5):312–7.
Roslin MS, Dudiy Y, Weiskopf J, et al. Comparison between RYGB, DS, and VSG effect on glucose homeostasis. Obes Surg. 2012;22(8):1281–6.
Abraham A, Ikramuddin S, Jahansouz C, et al. Trends in bariatric surgery: procedure selection, revisional surgeries, and readmissions. Obes Surg. 2016;26(7):1371–7.
Khorgami Z, Shoar S, Andalib A, et al. Trends in utilization of bariatric surgery, 2010-2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13(5):774–8.
Ahmed B, King WC, Gourash W, et al. Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the Longitudinal Assessment of Bariatric Surgery (LABS) study. Surgery. 2018;164(4):774–83.
Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(5):997–1002.
Creange C, Jenkins M, Pergamo M, et al. Gastric band conversion to Roux-en-Y gastric bypass shows greater weight loss than conversion to sleeve gastrectomy: 5-year outcomes. Surg Obes Relat Dis. 2018;14(10):1531–6.
Celio AC, Wu Q, Kasten KR, et al. Comparative effectiveness of Roux-en-Y gastric bypass and sleeve gastrectomy in super obese patients. Surg Endosc. 2017;31(1):317–23.
Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–54.
Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–65.
Kalinowski P, Paluszkiewicz R, Wróblewski T, et al. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass-results of a randomized clinical trial. Surg Obes Relat Dis. 2017;13(2):181–8.
Lewis KH, Arterburn DE, Zhang F, Callaway K, Wallace J, Fernandez A, Ross-Degnan D, Wharam JF. Comparative Effectiveness of Vertical Sleeve Gastrectomy Versus Roux en y Gastric Bypass for Diabetes Treatment: A Claims-based Cohort Study. Ann Surg. 2019;12(10):1097.
Wainscoat JS, Hill AVS, Boyce AL, et al. Evolutionary relationships of human populations from an analysis of nuclear DNA polymorphisms. Nature. 1986;319(6053):491–3.
Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117(6):241–50.
Longo M, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20(9):2358.
Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30.
Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21(12):1443–55.
Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7(1):14–24.
Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239.
Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22.
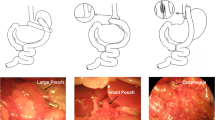
Stevenson M, Lee J, Lau RG, et al. Surgical mouse models of vertical sleeve gastrectomy and Roux-en Y gastric bypass: a review. Obes Surg. 2019;29(12):4084–94.
Hao Z, Zhao Z, Berthoud HR, et al. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS One. 2013;8(1):e52922.
Hao Z, Townsend RL, Mumphrey MB, et al. RYGB produces more sustained body weight loss and improvement of glycemic control compared with VSG in the diet-induced obese mouse model. Obes Surg. 2017;27(9):2424–33.
Frohman HA, Rychahou PG, Li J, et al. Development of murine bariatric surgery models: lessons learned. J Surg Res. 2018;229:302–10.
Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–9.
Surwit RS, Kuhn CM, Cochrane C, et al. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–7.
Spivak H, Sakran N, Dicker D, et al. Different effects of bariatric surgical procedures on dyslipidemia: a registry-based analysis. Surg Obes Relat Dis. 2017;13(7):1189–94.
Cunha FM, Oliveira J, Preto J, et al. The effect of bariatric surgery type on lipid profile: an age, sex, body mass index and excess weight loss matched study. Obes Surg. 2016;26(5):1041–7.
Kang YE, Kim JM, Joung KH, et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016;11(4):e0154003.
Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Futur Lipidol. 2008;3(5):545–56.
Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.
Han MS, White A, Perry RJ, et al. Regulation of adipose tissue inflammation by interleukin 6. Proc Natl Acad Sci U S A. 2020;117(6):2751–60.
Poret JM, Souza-Smith F, Marcell SJ, et al. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats. Int J Obes. 2018;42(3):535–41.
Zatterale F et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2019;10:1607.
Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55.
Yadav A, Kataria MA, Saini V, et al. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–4.
Soodini GR. Adiponectin and leptin in relation to insulin sensitivity. Metab Syndr Relat Disord. 2004;2(2):114–23.
Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83.
Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–703.
Akagiri S, Naito Y, Ichikawa H, et al. A mouse model of metabolic syndrome; increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J Clin Biochem Nutr. 2008;42(2):150–7.
Fruhbeck G et al. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7(1):57–62.
Hao Z, Mumphrey MB, Townsend RL, et al. Body composition, food intake, and energy expenditure in a murine model of Roux-en-Y Gastric bypass surgery. Obes Surg. 2016;26(9):2173–82.
Stanford KI, Middelbeek RJW, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–23.
He R, Yin Y, Li Y, et al. Esophagus-duodenum gastric bypass surgery improves glucose and lipid metabolism in mice. EBioMedicine. 2018;28:241–50.
Gong DW, He Y, Karas M, et al. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, beta3-adrenergic agonists, and leptin. J Biol Chem. 1997;272(39):24129–32.
Ben-Zvi D, Meoli L, Abidi WM, et al. Time-dependent molecular responses differ between gastric bypass and dieting but are conserved across species. Cell Metab. 2018;28(2):310–23. e6
Hernansanz-Agustin P et al. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature. 2020;586(7828):287–91.
Millan J et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–65.
Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin N Am. 2003;32(4):855–67.
Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;84(1A):28J–32J.
Chen Y, Yang J, Nie X, et al. Effects of bariatric surgery on change of brown adipocyte tissue and energy metabolism in obese mice. Obes Surg. 2018;28(3):820–30.
Heffron SP, Parikh A, Volodarskiy A, et al. Changes in lipid profile of obese patients following contemporary bariatric surgery: a meta-analysis. Am J Med. 2016;129(9):952–9.
Hsu SY, Lee WJ, Chong K, et al. Laparoscopic bariatric surgery for the treatment of severe hypertriglyceridemia. Asian J Surg. 2015;38(2):96–101.
Santos J, Salgado P, Santos C, et al. Effect of bariatric surgery on weight loss, inflammation, iron metabolism, and lipid profile. Scand J Surg. 2014;103(1):21–5.
Cancello R, Zulian A, Gentilini D, et al. Permanence of molecular features of obesity in subcutaneous adipose tissue of ex-obese subjects. Int J Obes. 2013;37(6):867–73.
Katsogiannos P, Kamble PG, Boersma GJ, et al. Early changes in adipose tissue morphology, gene expression, and metabolism after rygb in patients with obesity and T2D. J Clin Endocrinol Metab. 2019;104(7):2601–13.
Keidar A, Appelbaum L, Schweiger C, et al. Baseline abdominal lipid partitioning is associated with the metabolic response to bariatric surgery. Obes Surg. 2014;24(10):1709–16.
Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–8.
Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–5S.
Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3.
Andus T, Bauer J, Gerok W. Effects of cytokines on the liver. Hepatology. 1991;13(2):364–75.
Manco M, Mosca A, de Peppo F, et al. The benefit of sleeve gastrectomy in obese adolescents on nonalcoholic steatohepatitis and hepatic fibrosis. J Pediatr. 2017;180:31–7. e2
Whang E, Liu Y, Kageyama S, et al. Vertical sleeve gastrectomy attenuates the progression of non-alcoholic steatohepatitis in mice on a high-fat high-cholesterol diet. Obes Surg. 2019;29(8):2420–9.
Abu-Gazala S, Horwitz E, Ben-Haroush Schyr R, et al. Sleeve gastrectomy improves glycemia independent of weight loss by restoring hepatic insulin sensitivity. Diabetes. 2018;67(6):1079–85.
Kirk EA, Sagawa ZK, McDonald TO, et al. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes. 2008;57(5):1254–61.
Inouye KE, Shi H, Howard JK, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56(9):2242–50.
Wernstedt Asterholm I, Tao C, Morley TS, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103–18.
Kristof E et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp Cell Res. 2019;377(1-2):47–55.
Inoue M, Yano M, Yamakado M, et al. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism. 2006;55(9):1248–54.
Inoue M, Maehata E, Yano M, et al. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism. 2005;54(3):281–6.
Vega GL, Grundy SM. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes. 2013;2013:409679.
Fruhbeck G et al. Normalization of adiponectin concentrations by leptin replacement in ob/ob mice is accompanied by reductions in systemic oxidative stress and inflammation. Sci Rep. 2017;7(1):2752.
Fruhbeck G, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. 2019;11(2):454.
Unamuno X, et al. Increase of the adiponectin/leptin ratio in patients with obesity and type 2 diabetes after Roux-en-Y gastric bypass. Nutrients. 2019;11(9):2069.
Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–5.
Rajan S, Gupta A, Beg M, et al. Adipocyte transdifferentiation and its molecular targets. Differentiation. 2014;87(5):183–92.
Boss O, Farmer SR. Recruitment of brown adipose tissue as a therapy for obesity-associated diseases. Front Endocrinol (Lausanne). 2012;3:14.
Wang W, Ishibashi J, Trefely S, et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 2019;30(1):174–89. e5
Hankir MK, Seyfried F. Do bariatric surgeries enhance brown/beige adipose tissue thermogenesis? Front Endocrinol (Lausanne). 2020;11:275.
Jahansouz C, Serrot FJ, Frohnert BI, et al. Roux-en-Y gastric bypass acutely decreases protein carbonylation and increases expression of mitochondrial biogenesis genes in subcutaneous adipose tissue. Obes Surg. 2015;25(12):2376–85.
Lutz TA, Bueter M. The use of rat and mouse models in bariatric surgery experiments. Front Nutr. 2016;3:25.
Acknowledgements
We thank the animal care staff members for their help and kind support throughout the duration of the study.
Funding
This article is funded by The American Heart Association GIA Award #15GRNT22420001 and The George Link Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed and approved by NYU Long Island School of Medicine’s Institutional Animal Use and Care Committee, which adheres to guidelines provided by the National Institutes of Health.
Informed Consent
Informed consent does not apply.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 60 kb)
Rights and permissions
About this article
Cite this article
Stevenson, M., Srivastava, A., Lee, J. et al. RYGB Is More Effective than VSG at Protecting Mice from Prolonged High-Fat Diet Exposure: An Occasion to Roll Up Our Sleeves?. OBES SURG 31, 3227–3241 (2021). https://doi.org/10.1007/s11695-021-05389-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05389-8