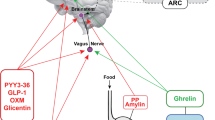
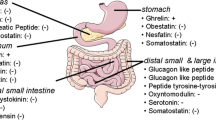
Abstract
Obesity is now considered the new world epidemic. In an attempt to face this menace to public health, several treatments, apart from the traditional nutritional modification and oral medication, have been introduced, among them bariatric surgery and gut hormone-based treatments. The gastrointestinal (GI) tract is a powerful endocrine organ, releasing active peptides and influencing appetite and glycaemic control. Alteration of the GI tract, in ways that exaggerate the secretion and levels of the gut hormones, creates a new functional equilibrium that further contributes to weight loss. The purpose of this review is to explore the mechanisms that drive this gut hormone-derived body regulation, as well as the changes that occur to them after bariatric surgery. Close to that, leptin, a hormone secreted by adipose tissue will be analysed, as its pathways are closely related to those of the gut hormones. Gut hormones are strongly implicated in energy control, and various effects of bariatric surgery in weight loss are directly related to the alteration of the levels of these hormones.


Similar content being viewed by others
Abbreviations
- AgRP:
-
Agouti-related peptide
- AMPK:
-
AMP-activated protein kinase
- ARC:
-
Arcuate nucleus
- α-MSH:
-
Alpha-melanocyte-stimulating hormone
- BMI:
-
Body mass index
- CNS:
-
Central nervous system
- CART:
-
Cocaine- and amphetamine-regulated transcript
- DPP4:
-
Dipeptidyl peptidase-4
- GHS-R:
-
Growth hormone secretagogue receptor
- GIP:
-
Glucose-dependent insulinotropic peptide
- GLP-1:
-
Glucagon-like peptide-1
- HbA1c:
-
Glycated haemoglobin
- HOMA-IR:
-
Homeostasis assessment model of insulin resistance
- NPY:
-
Neuropeptide Y
- NTS:
-
Nucleus of the solitary tract
- OXM:
-
Oxyntomodulin
- POMC:
-
Pro-opiomelanocortin
- PP:
-
Pancreatic polypeptide
- PVN:
-
Paraventricular nucleus
- PYY(3–36) :
-
Peptide YY(3–36)
- RYGB:
-
Roux-en-Y gastric bypass
References
Angelopoulos N, Goula A, Tolis G. Current knowledge in the neurophysiologic modulation of obesity. Metabolism. 2005;54(9):1202–17.
Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37.
Budak E, Sanchez F, Bellver J, et al. Interactions of the hormones leptin, ghrelin, adiponectin, resistin and PYY3-36 with the reproductive system. Fertil Steril. 2006;85(6):1563–81.
Lammert A, Kiess W, Bottner A, et al. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283(4):982–8.
Moreno-Aliaga MJ, Lorente-Cebrian S, Martinez JA. 3rd International Immunonutrition Workshop. Session 3: fatty acids and the immune system. Regulation of adipokine secretion by n-3 fatty acids. Proc Nutr Soc. 2010;69:324–32.
Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–75.
Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50(9):1511–25.
Havel PJ. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med. 2001;226:963–77.
Sinha MK, Ohannesian JP, Heiman ML, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–7.
Schoeller DA, Cella LK, Sinha MK, et al. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–7.
Hamnvik OP, Liu X, Petrou M, et al. Soluble leptin receptor and leptin are associated with baseline adiposity and metabolic risk factors, and predict adiposity, metabolic syndrome, and glucose levels at 2-year follow-up: the Cyprus Metabolism Prospective Cohort Study. Metabolism. 2011;60(7):987–93.
Gale SM, Castracane VD, Mantzoros CS. Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. J Nutr. 2004;134:295–8.
Lee YH, Magkos F, Mantzoros CS, et al. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism. 2011;60(12):1664–72.
Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801.
Westerterp-Plantenga MS, Saris WH, Hukshorn CJ, et al. Effects of weekly administration of pegylated recombinant human OB protein on appetite profile and energy metabolism in obese men. Am J Clin Nutr. 2001;74:426–34.
Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab. 2009;296:E1230–8.
Niswender KD, Morton GJ, Stearns WH, et al. Intracellular signalling: key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–5.
Sainsbury A, Cooney GJ, Herzog H. Hypothalamic regulation of energy homeostasis. Best Pract Res Clin Endocrinol Metab. 2002;16:623–7.
Magni P. Hormonal control of the neuropeptide Y system. Curr Protein Pept Sci. 2003;4:45–57.
Fernández-Riejos P, Najib S, Santos-Alvarez J, et al. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010;2010:568343.
Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53 Suppl 1:S143–51.
Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;137:2116–30.
Vincent RP, Ashrafian H, leRoux C. Mechanisms of disease: the role of gastrointestinal hormones in appetite and obesity. Nat Clin Pract Gastroenterol Hepatol. 2008;5:268–77.
Adrian TE, Ferri GL, Bacarese-Hamilton AJ, et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–7.
Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY (3–36) physiologically inhibits food intake. Nature. 2002;418:650–4.
Abbott CR, Small CJ, Kennedy AR, et al. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res. 2005;1043:139–44.
Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab. 2005;1:159–68.
Adrian TE, Savage AP, Sagor GR, et al. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89:494–9.
le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14.
Schonhoff S, Baggio L, Ratineau C, et al. Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY. Mol Cell Biol. 2005;25:4189–99.
Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–61.
Murakami N, Hayashida T, Kuroiwa T, et al. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174(2):283–8.
Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–8.
Cigaina V, Hirschberg AL. Plasma ghrelin and gastric pacing in morbidly obese patients. Metabolism. 2007;56(8):1017–21.
Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9.
Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13.
Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8.
Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–12.
Monteleone P, Bencivenga R, Longobardi N, et al. Differential responses of circulating ghrelin to high-fat or high carbohydrate meal in healthy women. J Clin Endocrinol Metab. 2003;88:5510–4.
Overduin J, Frayo RS, Grill HJ, et al. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–50.
Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8.
Kumar R, Salehi A, Rehfeld JF, et al. Proghrelin peptides: desacyl ghrelin is a powerful inhibitor of acylated ghrelin, likely to impair physiological effects of acyl ghrelin but not of obestatin A study of pancreatic polypeptide secretion from mouse islets. Regul Pept. 2010;164(2–3):65–70.
Gutierrez-Aguilar R, Woods SC. Nutrition and L and K-enteroendocrine cells. Curr Opin Endocrinol Diabetes Obes. 2011;18(1):35–41.
Wynne K, Park AJ, Small CJ, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390–5.
Dakin CL, Small CJ, Batterham RL, et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–95.
Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88:4696–701.
Wynne K, Park AJ, Small CJ, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes. 2006;30:1729–36.
Rijkelijkhuizen JM, McQuarrie K, Girman CJ, et al. Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism. 2010;59(4):502–11.
Creutzfeldt W, Ebert R, Willms B, et al. Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia. 1978;14:15–24.
Salera M, Giacomoni P, Pironi L, et al. Gastric inhibitory polypeptide release after oral glucose: relationship to glucose tolerance, diabetes mellitus and obesity. J Clin Endocrinol Metab. 1982;55:329–36.
Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signalling prevents obesity. Nat Med. 2002;8:738–42.
Holst JJ. On the physiology of GIP and GLP-1. Horm Metab Res. 2004;36:747–54.
Parkes DG, Pittner R, Jodka C, et al. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism. 2001;50(5):583–9.
Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf). 2008;69:173–9.
Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–6.
Naslund E, Barkeling B, King N, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–11.
Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72.
Verdich C, Toubro S, Buemann B, et al. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–14.
Burcelin R, Serino M, Cabou C. A role for the gut-to-brain GLP-1-dependent axis in the control of metabolism. Curr Opin Pharmacol. 2009;9:744–52.
Cabou C, Campistron G, Marsollier N, et al. Brain GLP-1 regulates arterial blood flow, heart rate and insulin sensitivity. Diabetes. 2008;57:2577–87.
Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110:571–84.
Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–85.
Korner J, Bessler M, Inabnet W, et al. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601.
Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–16.
Rodieux F, Giusti V, D’Alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring). 2008;16:298–305.
Reinehr T, Roth CL, Schernthaner GH, et al. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007;17:1571–7.
Clements RH, Gonzalez QH, Long CI, et al. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5.
Plum L, Ahmed L, Febres G, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring). 2011;19(11):2149–57.
Morinigo R, Vidal J, Lacy AM, et al. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–5.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–7.
le Roux CW, Neary NM, Halsey TJ, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90(8):4521–4.
Sundbom M, Holdstock C, Engström BE, et al. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–10.
Riedl M, Vila G, Maier C, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93:2307–12.
Faraj M, Jones P, Sniderman AD, et al. Enhanced dietary fat clearance in postobese women. J Lipid Res. 2001;42:571–80.
Molina A, Vendrell J, Gutierrez C, et al. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615–21.
Vendrell J, Broch M, Vilarrasa N, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–71.
Lee WJ, Chen CY, Chong K, et al. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7(6):683–90.
Mingrone G, Nolfe G, Gissey GC, et al. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia. 2009;52:873–81.
Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–15.
Holdstock C, Zethelius B, Sundbom M, et al. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond). 2008;32:1640–6.
Falkén Y, Hellström PM, Holst JJ, et al. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96(7):2227–35.
Kawada T. Preliminary report: homeostasis model assessment of insulin resistance, an indicator of insulin resistance, is strongly related to serum insulin: practical data presentation and the mathematical basis. Metabolism. 2010;59(7):1044–6.
Barker KB, Palekar NA, Bowers SP, et al. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2004;101:368–73.
Tiikkainen M, Bergholm R, Vehkavaara S, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–7.
Werner U, Haschke G, Herling AW, et al. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept. 2010;164:58–64.
Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2009;33:1255–61.
Ratner R, Nauck M, Kapitza C, et al. Safety and tolerability of high doses of taspoglutide, a once-weekly human GLP-1 analogue, in diabetic patients treated with metformin: a randomized double-blind placebo-controlled study. Diabet Med. 2010;27:556–62.
Rosenstock J, Reusch J, Bush M, et al. Albiglutide Study Group. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–6.
Beglinger C, Poller B, Arbit E, et al. Pharmacokinetics and pharmacodynamic effects of oral GLP-1 and PYY3-36: a proof-of-concept study in healthy subjects. Clin Pharmacol Ther. 2008;84:468–74.
Day JW, Ottaway N, Patterson JT, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749–57.
Liu YL, Ford HE, Druce MR, et al. Subcutaneous oxyntomodulin analogue administration reduces body weight in lean and obese rodents. Int J Obes. 2010;1–11.
Asakawa A, Inui A, Kaga T, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–52.
Pittner RA, Moore CX, Bhavsar SP, et al. Effects of PYY[3–36] in rodent models of diabetes and obesity. Int J Obes Relat Metab Disord. 2004;28:963–71.
Schouten R, Rijs CS, Bouvy ND, et al. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251(2):236–43.
Schouten R, Wiryasaputra DC, van Dielen FM, et al. Long-term results of bariatric restrictive procedures: a prospective study. Obes Surg. 2010;20(12):1617–26.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michalakis, K., le Roux, C. Gut Hormones and Leptin: Impact on Energy Control and Changes After Bariatric Surgery—What the Future Holds. OBES SURG 22, 1648–1657 (2012). https://doi.org/10.1007/s11695-012-0698-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0698-9