Abstract
The current study aimed to investigate the protection role of nanocomposite polypropylene films containing clay nanoparticles (NPs) on grated carrot. The effects of clay NPs (1 and 3%) were studied during storage (1, 3, 6, 9 and 12 days) in a completely randomized design. All samples were stored at 4 ± 1 °C. The chemical characteristics, color (L*, b* and a* values), sugars content (glucose, fructose and sucrose), total phenolic content and sensory qualities of packaged carrot were measured. Moreover, microbial counts namely E. coli, molds, yeasts, psychotropic bacteria as well as total count were determined. According to the results, application of clay NPs significantly (P < 0.01) reduced the pH, color indices as well as acidity. Nanocomposites with 3% NPs showed the highest total phenolic content (P < 0.01). Clay NPs also could significantly (P < 0.01) prevent the reduction of glucose in all samples. According to the microbial results, yeasts, molds and psychotropic bacteria were reduced during the 12 day storage. Therefore, it could be concluded that a decontamination step followed by packaging in nanocomposites can remarkably preserve the physicochemical and microbial characteristics as well as sensory quality of grated carrots during storage.
Similar content being viewed by others
Introduction
Fruit and vegetable consumption is necessary for a balanced diet. Increase in fresh-cut vegetable consumption challenges suppliers to deliver fresh products more quickly and cost-effectively [1]. Since carrot (Daucus carota L.) is one of the frequently consumed vegetables, many researches have focused on various aspects of such products. Some carrot treatments such as peeling or cutting, cause increase in tissue damage, respiration, color changes, loss of fresh-like flavor and, as a consequence, shelf life reduction. Since rapid deterioration reduces storage time, numerous methods and technologies have been introduced to extend the shelf life of minimally processed products [2,3,4,5,6].
The methods of food disinfection include application of organic acids [7], organic salts, electrolyzed water [2], high pressure [8], warm water [9] or steam blanching [7] to prevent enzyme activity, immersion in acid or basic solution [10], ozone [11], modified/controlled atmosphere [12] and application of edible films [13]. Besides, Propionic, acetic, malic, citric, lactic and tartaric acid may be used for food disinfection purposes [4, 10, 14]. US Food and Drug Administration (FDA) approves the use of peroxyacetic acid (PAA) as a strong oxidant for sanitizing some food products like fruits and vegetables (concentration of PPA in washing water should not exceed 80 ppm) [15]. Low temperature during storage and atmospheric conditions including 3–8% CO2 and 2–5% O2 are recommended to maintain the freshness and extending fruits and vegetables shelf life for modified atmosphere packaging (MAP) storage [14, 16, 17].
Application of nanotechnology can not only improve physical, chemical and microbial characteristics but also decrease the costs of products remarkably. Layered inorganic solids such as clays and silicates are interesting for packaging industry because of their accessibility, low price, and rather plain processability [16, 18, 19]. Vandekinderen et al. (2009) investigated the effects of peroxyacetic acid decontamination on microbial and chemical characteristics of grated carrot and found that under the given conditions, 80 mg L−1 peroxyacetic acid resulted in the enhancement of carrot shelf life without any deep impact on their nutritional profile [10]. Likewise, Guinamas et al. (2015), studied physicochemical and microbial characteristics of fresh cut carrots coated with starch- montmorillonite (MMT) nanofilms packed under modified atmospheric packaging and concluded that starch/montmorillonite films didn’t impact the respiration rate of carrots, but successfully diminished their weight loss [20]. Costa et al. (2013) evaluated calcium-alginate films loaded with Ag-MMT NPs and studied microbial characteristics of carrots during shelf life period[21] and drew a conclusion that a remarkable shelf life improvement of higher than 2 months could be obtained by applying calcium-alginate coating loaded with Ag-MMT NPs. In a more recent research, Barikloo and Ahmadi (2018), investigated the shelf life enhancement of strawberry using chitosan-clay-silica nanocomposites and modified atmospheric packaging through microbial assays and realized that incorporation of only 1% clay and 0.75% silver could notably improve physical, chemical and mechanical characteristics of the strawberry during its shelf life [22].
Based on the results of previous research, more detailed experiments on sugar content, phenolic compounds, sensory characteristics and microbial quality of fresh cut and grated carrots seem to be necessary due to their high consumption in the field of food industry. Consequently, the objective of the current study was to investigate the effects of polypropylene and low density poly ethylene films containing various percentages of Nano bentonite on the microbial quality and shelf life of the minimally processed carrots during 12 days of cold storage.
Material and methods
Plant material
30 kg fresh carrots were obtained from a carrot field (Esfahan, Iran) and transferred to the laboratory at refrigerated (4 °C) condition within 1 h. The carrots were washed twice using sterile water to diminish any dust and silt. Carrots were peeled and cut into small sticks using cutting machine and washed for the third time by sterile water to reduce potential contaminations.
Preparation of peroxyacetic acid
Peroxyacetic acid was purchased from Merck (Germany). Iodometric titration was used to determine the precise concentration of peroxyacetic acid in obtained solution. 80 mg/L solution was prepared according to Vandekinderen et al. [10]. To prepare packaging, grated carrots were immersed using 1:10 (w/v) solution and mixed for 10 min. Finally, excessive amount of sanitizer was removed using manual centrifugation (600 rpm, 30 s; Isfahan Corporation, Iran).
Production of polypropylene films and packaging
Various percentages (1% and 3% w/w) of hydrophobic nano-bentonite (Merck, Germany) were added to polypropylene granules followed by mixing to prepare a homogenous sample. Nanofilms were produced using melt mixing method by applying four different heating zones (145, 158, 167, 177 °C). Produced samples were kept at sterile condition for further analysis. [23, 24].
According to Vandekinderen et al. (2009), equilibrium modified atmospheric packaging using 5% O2, 5% CO2 and 90% N2 were applied. Oxygen permeability of produced bentonite nanofilms were 0.035 nmol O2 nmol−2 s−1 Pa−1. The length and width of used films were 250 mm and 150 mm, respectively.
Determination of physicochemical properties during shelf life
To study the physicochemical, grated carrots were packed into various (0, 1 and 3%) packaging. Sampling was performed at three days intervals during 12 days of experiments (1, 3, 6, 9, 12 and 15 days). 1 packaging was assigned for each day of experiment. Firstly, weight loss was specified for each packaging, then microbial characteristics of stored carrots were evaluated under aseptic conditions. Total soluble content (TSC), pH, acidity, total phenolic compounds, sugar content and colorimetric assays were investigated.
pH and acidity
The grated carrots were homogenized in distilled water (1:1, w/v) at room temperature for pH determination (CP501, Elmetron, Poland). The titrable acidity (TA) was determined by titrating 10 g of a homogenized sample in 80 mL of water containing 0.1 mol/L NaOH (pH 8.1). A potentiometric titrator system was applied for this purpose. The results were expressed as malic acid percentage [11].
Total soluble solid content
The total soluble solid (TSS) content was determined by a portable refractometer (BPTR-10`0, PrismaTech; Benchtop Refractometer, Iran) and reported as ◦Brix. A digital precision balance was used for the sample’s weight loss calculation (DV-H, Mettler Toledo, Swiss) [11, 12].
Colorimetric assays
The color parameters of grated carrot were determined by Hunter-Lab color meter (Hunter Lab CR-400 color difference meter, Konica Minolta, Japan). Lightness to darkness (L*), redness to greenness (a*) and yellowness to blueness (b*) were quantified at least in four different points of each pack. Total color differences (ΔE*) as presented in Eq. 1 was used to evaluate color changes during storage time.
where \({\mathrm{L}}_{0}^{*}\), \({\mathrm{a}}_{0}^{*}\) and \({\mathrm{b}}_{0}^{*}\) values are colour parameters of the fresh sample on the first day of storage and L*, a* and b* values are color parameters of the fresh sample on the storage days.
The whiteness index (WI) as presented in Eq. 2 was calculated for carrots:
Phenolic compounds measurements
The extraction procedure for total phenolics described by Vinson, Hao, Su, and Zubik (1998) was used to determine the total phenol content. A sample (2 g) was diluted to 20 mL with HCl (1.2 M) in aqueous methanol (50%).The mixture was shaken for 2 h at 90 °C and filtered. The filtrates were prepared in triplicates and stored at – 20 °C. The storage time was not more than 24 h. The Folin–Ciocalteu method was used to determine the total phenol content. The filtrate (2 mL) was mixed with 5 mL of Folin–Ciocalteu reagent (10 times diluted) and after 6 min (± 10 s), 15 mL of 20% Na2CO3 (w/v) was added to the mixture. Then, the mixture was diluted to 100 mL with distilled water and shaken for 2 h at room temperature in the dark. A wavelength of 760 nm was used for absorbance reading of samples against blank. Gallic acid standard solution (0–400 mg/L) was applied to depict a standard curve for the total phenolic contents. An aliquot of 1 mL gallic acid solution was used under the same procedure explained above. The total phenolic contents were expressed as mg gallic acid kg−1 of fresh carrot [12, 25].
Sugar measurement
Three packaging per treatment were selected to determine the sugar content of samples during storage. The extraction procedure described by Dokhani et al. (1988) was used for sugar measurement. The carrots samples (10 g) were homogenized, added to 0.02 g ethylene diamine tetra acetic acid (EDTA). The mixture was brought to the volume of 100 mL by sodium azide (0.1%), then, centrifuged at 3000 rpm for 15 min, and filtrated through a 0.45 µm syringe filter. The procedure described by Klaiber et al. (2005) was followed with some modifications [9].
For analysis of glucose, fructose, and sucrose, a Waters HPLC system (HP 1090-ΙΙ liquid chromatograph; now Agilent, Palo Alto, CA, USA)) equipped with a refractive index detector (RID-6A, Shimadzu, Japan) was applied. The separation was performed on a Hector-M-(NH2) an YMC Polyamine II column (150 mm × 3 mm i.d.) with a particle size of 3 µm (RStech Co, Daejean, Korea) fitted with an YMC Polyamine II pre-column (10 mm × 4 mm i.d.) (RStech Co, Daejean, Korea) with a particle size of 5 µm. Glucose, fructose, and sucrose were quantified by external standard calibration. The total sugar contents were calculated as the sum of fructose, glucose, and sucrose.
Microbiological experiments during shelf life
Preparation of inoculation
Two strains of Escherichia coli O157:H7 (ATCC 25932 and ATCC 25933) were obtained from the Scientific and Industrial Research Organization of Iran. The stock cultures were maintained on tryptic soy agar (TSA) slants at 4 °C. Prior to the use, each strain was separately cultured in Tryptic Soy Broth (TSB) at 37 °C with two consecutive transfers after 24 h periods for a total of 48 h of incubation. All working cultures (109 CFU/mL) cultured in TSB, were separately centrifuged at 4000×g for 15 min at 4 °C and the supernatants were discarded. The cell pellets were washed twice using 0.1% peptone water (pH 7.1) and mixed in 10 mL of the same solution to establish concentration of 109 CFU/mL [26]. The bacterial crowd in each cocktail culture was authenticated by plating 0.1 mL portions of appropriately diluted culture on TSA. Since the immersion process is a probable contamination location in the food industry, dip inoculation is the most appropriate procedure to imitate such an operation [15]. The grated carrots were immersed in the bacterial inoculum solution (inoculum ratio D1:5 w/v) and stirred for 2 min. After dipping, the samples were drained for 30 s and spin-dried for 30 s in a manually activated salad spinner (Metro Essoreuse, CA). Then, 1 g of each sample was inoculated with E. coli, wrapped using nanofilms (5 cm × 5 cm) and stored at 4 °C for 15 days. To reduce cross contamination by refrigerator’s ventilation system, each packaging was kept in a protected petri dish. Finally, E. coli colony count was carried out on 1, 3, 5, 7, 10, 12 and 15 days of storage.
Mold-yeast and psychotropic counts
Periodically, 1 g of each sample was aseptically removed from each packaging, placed in a test tube, diluted with 0.9% (w/v) peptone water solution and homogenized using a stomacher blender 400 (Pbi International, Italy). Serial dilution was carried out using sterile peptone water solution; then, samples were plated onto appropriate media. Plate Count Agar (PCA) incubated at 7 °C for 10 days for psychotropic bacteria; Sabouraud dextrose agar, supplemented with chloramphenicol (0.1 g/L) (C. Erba, Italy), incubated at 25 °C for 48 h, for mold-yeast test [27].
E. coli counts
To measure E. coli count, 1 g of each sample was inoculated with E. coli and placed in a test tube diluted with 0.9% (w/v). Peptone water solution was homogenized using a stomacher blender 400 (Pbi International, Italy). After the homogenization step, 1 g of the sample was diluted in 9 mL of sterile Peptone water solution and 0.7 mL of sample was spread onto each selective media. The selective media, Eosin methylene-blue agar (EMB) were used for enumeration of E. coli O157:H7. Finally, the plate was incubated at 37 °C for 24 h. Obtained results were expressed as the log CFU/g [28, 29].
Analysis of NPs and synthesized nanofilms
To evaluate size, shape and coagulation status of NPs after incorporation in the film Scanning Electron Microscopy (SEM) was carried out using Hitachi Device (Japan) at 40 KX and 40 kV [30].
Evaluation of sensory characteristics
The sensory evaluation of grated carrots packed in polypropylene (control samples) and polypropylene containing 1 and 3 percent nano-clay hydrophilic bentonite was performed by 10 panelists on storage days 1, 3, 6, 9 and 12. A five point numeric rating scale was used for color, fresh-like aroma, sweetness, overall visual quality, and overall acceptance [11].
Statistical analysis
The experiments were performed in a factorial form, using a completely randomized design with three replications. Statistical analyses were conducted using SAS version 9.1.2 from SAS Institute, Inc., Cray, NC, USA. Data were reported as means ± standard deviation (SD). Data were subjected to analysis of variance (ANOVA) and Duncan’s multiple range method was used for comparisons of the means. Probability level of 1% was considered for data fitting in this study.
Results and discussion
Morphology of the prepared films
According to the results of scanning electron microscopy (Fig. 1), nano bentonite particle size was 40–50 nm in LDPE packaging. Application of nanoparticles < 100 nm can remarkably increase antimicrobial characteristics of nanoparticles due to increasing the volume to surface ratio. Therefore, since measured size of clay nanoparticles were revealed to be 40–50 nm, antimicrobial characteristics as well as increasing gas barrier properties were expected [16].
Physicochemical measurements
The changes of pH values and acidity (%) during storage are shown in Fig. 2. Some significant differences between the control samples and the samples in nanopackaging containing 1 and 3% nanoclay hydrophilic bentonite were observed regarding the pH values (P < 0.01). In general, the pH values for almost all the treatments remained constant until day 9 then slight decrease was observed by day 12 (Fig. 2). Decrease of pH may be due to aerobic bacteria activity, microbial growth in anoxic conditions and acidic metabolites production [31]. Acidity (%) changes were in agreement with pH decreases for the carrots in all packages (Fig. 2). The control samples showed the highest pH and the lowest acidity at the end of the storage time (P < 0.01). In agreement with the current study, Vandekinderen et al. (2009) showed that decontaminated carrot samples showed a reduction of pH during 9 days of experiment [10].
Moisture loss followed by shrinkage is the main physical change during storage. Weight loss of less than 0.5%, as was observed in the present research, had no visible wilting symptoms [12]. In this study, the weight loss was less than 0.17% for all the samples. These results as shown in Fig. 3 were in agreement with the results of the appearance ratings, in the sensory evaluation [8].
The Total Solid Soluble (TSS) content of the carrot samples (Fig. 4) decreased (P < 0.01) by increasing both independent variables (time and nanoclay level in packaging film). However, The TSS losses in the control samples were higher (P < 0.01) than other samples (Fig. 4). Sugar consumption in metabolic activities may be the reason for the °brix decrease [32].
Sugars content
The harvest time, cultivar and growing conditions of the carrots affect their total and individual sugar content which is the main factor in their taste. Sucrose is more important in appearing the sweet taste of the carrots compared to glucose and fructose [12].
The changes in the sugar contents in the carrots from day 1 to 12 are presented in Table 1. The sugar changes during the 12-day storage period were significant (P < 0.01). The sucrose and fructose contents decreased but the glucose level increased. The sucrose content of carrots decreased in all the samples; but the grated carrots packed in polypropylene containing 1 and 3 percent nano-clay hydrophilic bentonite showed a higher decrease (P > 0.05) and a higher relative increase in glucose (P < 0.01) compared to the control samples.
The greatest reduction was observed in fructose level (P > 0.05) of the control sample. The films with low oxygen transmission rate (OTR) caused higher sugar loss during storage [9]. The films containing 3% Nano-bentonite caused less changes in glucose.
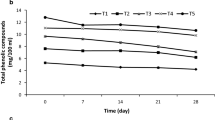
Total phenolic content variations
Phenolic compounds in different products display antioxidant activities (Lavelli et al., 2006). Post- harvest stresses cause phenolic compounds synthesis such as lignin produced at the plant wound to protect it from microbial and oxidative damage [33]. The storage time significantly (P < 0.01) affected the concentration of all phenolic compounds (Fig. 5). The phenolic compounds in all treatments kept decreasing until the third day, then got increased and remained constant until the end of the storage period (Fig. 5). At the end of the storage period, the grated carrots packed in polypropylene containing 1 and 3 percent nano-clay hydrophilic bentonite showed higher levels of phenolic compounds (P < 0.01) compared to the control sample. The similar results have been reported by other researchers [10].
Colorimetry
Since, the color of the carrots is an important factor for consumers, the color of all samples were determined by calculating the lightness (L*), redness (a*), and yellowness (b*) (Table 2). The L* value significantly increased at the end of the shelf-life for all the samples (P < 0.01), indicating a change to the lighter color components. At the end of the storage period, the luminosity L* value was the lowest for the carrots packaged in polypropylene containing 3 percent nano-clay hydrophilic bentonite, intermediate for the carrots packaged in polypropylene containing 1% nano-clay hydrophilic bentonite and the highest for the control samples. At the end of the shelf-life, the a* value of the carrots packaged in polypropylene containing 3 percent nano-clay hydrophilic bentonite was significantly higher than the a* value of the other treatments, indicating more red-color components (P < 0.05). β-carotene content of the carrots is a nutrient appreciated by consumers and correlates with the redness [34]. The b* value of the carrots packaged in polypropylene containing 3 percent nano-clay hydrophilic bentonite was significantly lower than the b* value of the other series (P < 0.01), indicating decrease in yellowness. The color changes during shelf life periods can be seen at Fig. 6.
Dehydration of the carrot samples during storage and lignin development caused increase in whiteness index (15,18). The grated carrots packaged in polypropylene containing nano-clay hydrophilic bentonite showed lower (P < 0.05) WI* values comparing to the control samples which were in agreement with the results obtained for L* values, and indicated an effective control over development of surface discoloration on the grated carrots. Since whiteness and redness of carrots are important factors to determine their shelf life, Emmambux and Minnaar (2003) reported that packaging films retaining moisture better are preferred [13]. During the whole shelf-life study, ΔE was the lowest for the carrots packaged in polypropylene containing 3 percent nano-clay hydrophilic bentonite and the highest for the control samples (Fig. 6).
As Eqs. 2 and 3 show WI and delta E are functions of different parameters of L*, a*, and b*. These parameters may change according to different chemical and microbiological variables. For example, some phenolic compounds like caffeic, coumaric, syringic acid are the main intermediaries of lignin synthesis pathway. Reduction of these precursors indicate polymerization of these phenolic acids to lignin and lignin increase has correlation to whiteness index. On the other hand, changes in total soluble solids and total sugars and shrinkage due to water loss during storage have some effects on Lightness of samples. Microbial changes specially yeast and mold growth on the surface of samples and production of water in their growth process have some effects on L. different concentration and accumulation of flavonoids may also have correlation with whiteness [35].
Microbial analysis
The effect of packaging films on the mold and yeast population
According to the results, no mold or yeast growth was observed until the 5th day of experiments. Apparently, using peroxyacetic acid has prevented their growth due to its extreme oxidizing properties. Fresh products usually have living tissues and breathe. Thus, their oxygen is consumed to produce carbon dioxide and volatile compounds such as alcohol and short-chain fatty acids. Knowing the fact that carbon dioxide is anti-fungal and anti-bacterial, the significant reduction in the growth of mold and yeast in the package containing clay nanoparticles compared to the control samples can be attributed to the accumulation of carbon dioxide inside the packaging [36].
Comparison of the samples containing different percentage of nanoparticles with the control sample showed the highest increase in the growth of mold and yeast on the day of the experiment. The films containing nanoparticles had significantly lowered mold and yeast growth. The Variance showed that the percentage of nanoparticles, time and also their interaction had significant effects on the population of mold and yeast (P < 0.01).
The effect of packaging film type on the psychrotrophs population
Based on the results of analysis of variance and Fig. 7, the percentage of nanoparticles and time significantly affected psychotrophs population changes (P < 0.01). A significant reduction was observed in the control samples by the 5th day while packaging containing nano-bentonite reduced bacterial load by the 10th day of the experiment. Nitrogen is proved to be inert, having no antibacterial effect; however it provides an anaerobic environment to prevent growth of aerobic microorganisms and delays fat oxidation [37]. Thus, using a combination of 90% nitrogen in modified atmosphere showed a decline in the growth of psychotropic bacteria in all samples. The highest growth of psychrotrophs in the storage period was related to the control sample.and the lowest was in relevant to the packaging containing 3% nano-clay.
Effect of nanofilms on E. coli
The results of E. coli colony counting are given in Fig. 7c. As observed, in all the packaging E. coli has raised on the 3th day of the experiment. Most spoilage bacteria and mold require oxygen for growth; therefore, the shelf life of packaged foods is increased in low concentrations of oxygen [38]. On the day 5, the E. coli count was reduced in all of the three treatments. That seemed to be the result of breathing of the product as well as conversion of oxygen to carbon dioxide. Liquid and gaseous carbon dioxide has antifungal and antibacterial properties, but not bactericidal. Carbon dioxide prevents bacterial growth by increasing the lag phase and reduces the growth rate in logarithmic phase. CO2 has the greatest impact on the food in which aerobic microorganisms and pathogenic Gram-negative bacteria are the spoilage factors. E. coli growth in the control sample was three times higher and in packages containing 1% nano-bentonite, 1.7 times as high as the growth of E. coli in packages containing 3% nano-bentonite. Studies showed that antimicrobial effects of nanoparticles on the tested Gram-negative microorganisms is more than Gram-positive, which is due to the thick peptidoglycan walls of the microorganisms and its protective effect on the cell survival [39].
Results showed that the factors of percentage of time, nanoparticles percentage, and interaction between these two, significantly affected E. coli population decrease at the probability level of 1%. The treatment containing 3% of nanoparticles performed slightly better at the end of the maintenance period, probably due to the carbon dioxide increase, as a result, oxygen and breathing rate decreased and antibacterial feature increased.
Sensory analysis
The carrot samples were evaluated by 10 trained panelists for the sensory attributes (color, fresh-like appearance, fresh-like aroma and overall acceptance). For this purpose, a 5-point numeric scale was applied. Score 3 was considered for overall acceptance—a criterion for marketability limit-. End of shelf life was the time at least one of the mean scores got higher than 3 [27]. Based on the obtained results, the sensory properties did not score 3 after 12 days of storage at 4 °C, indicating a good sensory quality. The color rating of all the samples showed no significant differences during storage (P < 0.05). However, the color scores were higher for those carrots in polypropylene film containing 1 percent nano-clay hydrophilic bentonite, indicating the darker appearance of these samples. At the end of the shelf-life (day 12), only the grated carrots in polypropylene film containing 3% nano-clay scored better than the other two samples for fresh-like aroma (P > 0.05) because the 3%Nano film maintained the modified atmospheric condition better than the other treatments [27]. Off-flavors, microbial growth, softening and slime production are observed in anaerobic catabolism [32].
The off-flavor was developed in the grated carrots after 12 days of storage, indicating the end of shelf life. Firmness, juiciness, Sweetness, flavor, and freshness are important factors for consumers [27]. In this study, the flavor and sweetness scores were in good agreement for the carrot samples in all packs and this trend remained constant during the storage. However, the off-flavor scores increased over time, and were slightly higher for the control samples. The appearance changes were not significant for the packages (P < 0.05). However, the sample in polypropylene and LDPE films containing 1% and 3% nano-clay hydrophilic bentonite had a better appearance than other samples; Also, the visual appearance was better at the beginning of storage period than at the end of experiment. Finally, the overall acceptability was high and remained constant throughout the testing and storage period.
The results indicate that grated carrots in polypropylene film containing 1 and 3% nano-clay hydrophilic bentonite did not diminish the overall sensory quality compared to the control samples and the differences perceived in the packaging procedures were not significant during storage. However, the majority of the panelists indicated that the grated carrots packaged in polypropylene film containing 1 and 3% nano-clay were acceptable for consumption after 12 day of storage (Data not shown).
Conclusion
Packaging containing Nano bentonite was remarkably effective in maintaining the grated carrots quality and sensory attributes. The produced films containing Nano bentonite were beneficial in providing a better color retention. Results revealed that the overall acceptability of grated carrots packaged in polypropylene containing 1 and 3% nanoclay hydrophilic bentonite could maintain the sensory characteristics of grated carrots during shelflife. According to the results of physicochemical assays, decontamination step followed by application of nano clay packaging maintained sugar content as well as weight loss in a favorable level. Microbial results revealed that application of nanoclay could significantly increase the shelf life of grated carrot (P < 0.05) A 12-day shelf life was suggested for the minimally processed carrots for film containing 3% Nano bentonite comparing to 5 days in the previous research. Therefore, application of film containing nano bentonite could be recommended to extend shelf life of carrots.
References
P.A. Picouet, C. Sárraga, S. Cofán, N. Belletti, M.D. Guardia, Effects of thermal and high-pressure treatments on the carotene content, microbiological safety and sensory properties of acidified and of non-acidified carrot juice. LWT-Food Sci. Technol. 62(1), 920–926 (2015)
S. Rahman, Y.-G. Jin, D.-H. Oh, Combination treatment of alkaline electrolyzed water and citric acid with mild heat to ensure microbial safety, shelf-life and sensory quality of shredded carrots. Food Microbiol. 28(3), 484–491 (2011)
H. Liu, D. Li, W. Xu, Y. Fu, R. Liao, J. Shi, Y. Chen, Application of passive modified atmosphere packaging in the preservation of sweet corns at ambient temperature. LWT. 136(1), 110295 (2021)
S. Maqsood, S. Benjakul, Preventive effect of tannic acid in combination with modified atmospheric packaging on the quality losses of the refrigerated ground beef. Food Control 21(9), 1282–1290 (2010)
Z. Moslehi, A. Mohammadi Nafchi, M. Moslehi, S. Jafarzadeh, Aflatoxin, microbial contamination, sensory attributes, and morphological analysis of pistachio nut coated with methylcellulose. Food Sci. Nutr. (2021). https://doi.org/10.1002/fsn3.2212
D. Mousavian, A.M. Nafchi, L. Nouri, A. Abedinia, Physicomechanical properties, release kinetics, and antimicrobial activity of activated low-density polyethylene and orientated polypropylene films by Thyme essential oil active component. J. Food Meas. Charact. 15(1), 883–891 (2021)
D. Bermúdez-Aguirre, G.V. Barbosa-Cánovas, Disinfection of selected vegetables under nonthermal treatments: chlorine, acid citric, ultraviolet light and ozone. Food Control 29(1), 82–90 (2013)
X. Bi, J. Wu, Y. Zhang, Z. Xu, X. Liao, High pressure carbon dioxide treatment for fresh-cut carrot slices. Innov. Food Sci. Emerg. Technol. 12(3), 298–304 (2011)
R.G. Klaiber, S. Baur, G. Wolf, W.P. Hammes, R. Carle, Quality of minimally processed carrots as affected by warm water washing and chlorination. Innov. Food Sci. Emerg. Technol. 6(3), 351–362 (2005)
I. Vandekinderen, F. Devlieghere, J. Van Camp, Q. Denon, S.S. Alarcon, P. Ragaert, B. De Meulenaer, Impact of a decontamination step with peroxyacetic acid on the shelf-life, sensory quality and nutrient content of grated carrots packed under equilibrium modified atmosphere and stored at 7 C. Postharvest Biol. Technol. 54(3), 141–152 (2009)
C. Alegria, J. Pinheiro, E.M. Gonçalves, I. Fernandes, M. Moldão, M. Abreu, Quality attributes of shredded carrot (Daucus carota L. cv. Nantes) as affected by alternative decontamination processes to chlorine. Innov. Food Sci. Emerg. Technol. 10(1), 61–69 (2009)
H. Larsen, A.-B. Wold, Effect of modified atmosphere packaging on sensory quality, chemical parameters and shelf life of carrot roots (Daucus carota L.) stored at chilled and abusive temperatures. Postharvest Biol. Technol. 114, 76–85 (2016)
N.M. Emmambux, A. Minnaar, The effect of edible coatings and polymeric packaging films on the quality of minimally processed carrots. J. Sci. Food Agric. 83(10), 1065–1071 (2003)
S. Jafarzadeh, F. Ariffin, S. Mahmud, A. Najafi, M. Ahmad, Fabrication and characterization of novel semolina-based antimicrobial films derived from the combination of ZnO nanorods and nanokaolin. J. Food Sci. Technol. 54(1), 105–113 (2017)
S. Ruiz-Cruz, E. Acedo-Félix, M. Díaz-Cinco, M.A. Islas-Osuna, G.A. González-Aguilar, Efficacy of sanitizers in reducing Escherichia coli O157: H7, Salmonella spp. and Listeria monocytogenes populations on fresh-cut carrots. Food Control 18(11), 1383–1390 (2007)
R. Hosseini, H. Ahari, P. Mahasti, S. Paidari, Measuring the migration of silver from silver nanocomposite polyethylene packaging based on (TiO2) into Penaeus semisulcatus using titration comparison with migration methods. Fish. Sci. 83(4), 649–659 (2017)
A. Kita, S. Lachowicz, P. Filutowska, Effects of package type on the quality of fruits and nuts panned in chocolate during long-time storage. LWT (2020). https://doi.org/10.1016/j.lwt.2020.109212
D. Dehnad, H. Mirzaei, Z. Emam-Djomeh, S.-M. Jafari, S. Dadashi, Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 109, 148–154 (2014)
S. Paidari, S.A. Ibrahim, Potential application of gold nanoparticles in food packaging: a mini review. Gold Bull. (2021). https://doi.org/10.1007/s13404-021-00290-9
I. Guimarães, E. Menezes, P. Borges, R. Leal, K. Reis, B.E. de Vilas Boas, Fresh-cut carrot coated with starch/montmorillonite films and subjected to modified atmosphere packaging. In: XI International Controlled and Modified Atmosphere Research Conference, vol 1071 (2013), pp. 559–566
C. Costa, A. Conte, G. Buonocore, M. Lavorgna, M.A. Del Nobile, Calcium-alginate coating loaded with silver-montmorillonite nanoparticles to prolong the shelf-life of fresh-cut carrots. Food Res. Int. 48(1), 164–169 (2012)
H. Barikloo, E. Ahmadi, Shelf life extension of strawberry by temperatures conditioning, chitosan coating, modified atmosphere, and clay and silica nanocomposite packaging. Sci. Horticult. 240, 496–508 (2018)
H. Ahari, G. Karim, S.A. Anvar, S. Paidari, S.A. Mostaghim, A.S. Mazinani, Method for producing antimicrobial nanofilms packaging cover based on titanium nano-dioxide through extrusion for extension of food shelf-life. Google Patents (2020)
S. Jafarzadeh, A. Salehabadi, S.M. Jafari, 10 Metal nanoparticles as antimicrobial agents in food packaging. Hand Book of Nanotechology, Elsevier (2020)
S. Jafarzadeh, S.M. Jafari, Impact of metal nanoparticles on the mechanical, barrier, optical and thermal properties of biodegradable food packaging materials. Crit. Rev. Food Sci. Nutr. (2020). https://doi.org/10.1080/10408398.2020.1783200
R. Klaiber, S. Baur, L. Magel, W. Hammes, R. Carle, Quality of shredded, packaged carrots as affected by different washing treatments. J. Food Sci. 69(4), SNQ161–SNQ166 (2004)
C. Alegria, J. Pinheiro, E.M. Gonçalves, I. Fernandes, M. Moldão, M. Abreu, Evaluation of a pre-cut heat treatment as an alternative to chlorine in minimally processed shredded carrot. Innov. Food Sci. Emerg. Technol. 11(1), 155–161 (2010)
A. Anvar, S. Haghigha tKajavi, H. Ahari, A. Sharifan, A. Motallebi, S. Kakoolaki, S. Paidari, Evaluation of the antibacterial effects of Ag-TiO2 nanoparticles and optimization of its migration to sturgeon caviar (Beluga). Iran. J. Fish. Sci. 18(4), 954–967 (2019)
H. Ahari, M. Alinejad Dizaj, S. Paidari, A. Anvar, The effect of Gamma (γ) irradiation to inactivate Escherichia coli in contaminated water. Iran. J. Aquat. Anim. Health 2(2), 88–96 (2016)
Z. Wang, G. Li, G. Xie, Z. Zhang, Dispersion behavior of TiO2 nanoparticles in LLDPE/LDPE/TiO2 nanocomposites. Macromol. Chem. Phys. 206(2), 258–262 (2005)
S. Azlin-Hasim, M.C. Cruz-Romero, M.A. Morris, E. Cummins, J.P. Kerry, Effects of a combination of antimicrobial silver low density polyethylene nanocomposite films and modified atmosphere packaging on the shelf life of chicken breast fillets. Food Packag. Shelf Life 4, 26–35 (2015)
Z. Ayhan, O. Eştürk, E. Taş, Effect of modified atmosphere packaging on the quality and shelf life of minimally processed carrots. Turk. J. Agric. For. 32(1), 57–64 (2008)
J. Vaishnav, V. Adiani, P.S. Variyar, Radiation processing for enhancing shelf life and quality characteristics of minimally processed ready-to-cook (RTC) cauliflower (Brassica oleracea). Food Packag. Shelf Life 5, 50–55 (2015)
R.-u Haq, K. Prasad, Antioxidant activity, phenolic, carotenoid and color changes in packaged fresh carrots stored under refrigeration temperature. J. Food Meas. Charact. 11(4), 1542–1549 (2017)
K. Ranjitha, D.S. Rao, K. Shivashankara, H.S. Oberoi, T.K. Roy, H. Bharathamma, Shelf-life extension and quality retention in fresh-cut carrots coated with pectin. Innov. Food Sci. Emerg. Technol. 42, 91–100 (2017)
L. Ebrahimi, H. Etebarian, H. Aminian, N. Sahebani, Biological control of apple blue mold disease with Metschnikowia pulcherrima alone and in combination with silicon and its mechanisms of antagonism. Iran. J. Plant Pathol. 49(1), 123–129 (2013)
J.H. Han, Antimicrobial packaging systems, in Innovations in Food Packaging. (Elsevier, Amsterdam, 2005), pp. 80–107
J. Huang, Y. Guo, Q. Hou, M. Huang, X. Zhou, Dynamic changes of the bacterial communities in roast chicken stored under normal and modified atmosphere packaging. J. Food Sci. 85(4), 1231–1239 (2020)
S.H. Fasihnia, S.H. Peighambardoust, S.J. Peighambardoust, Nanocomposite films containing organoclay nanoparticles as an antimicrobial (active) packaging for potential food application. J. Food Process. Preserv. 42(2), e13488 (2018)
Acknowledgements
The authors are thankful to Matin Sabz Zayanderud (Saida) industries for allowing the experimental work to be performed at their site.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghorbani, Z., Zamindar, N., Baghersad, S. et al. Evaluation of quality attributes of grated carrot packaged within polypropylene-clay nanocomposites. Food Measure 15, 3770–3781 (2021). https://doi.org/10.1007/s11694-021-00925-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00925-7